Thoracentesis
| Thoracentesis | |
|---|---|
| Other names: Pleural tap, catheter thoracostomy, thoracocentesis, paracentesis thoracis | |
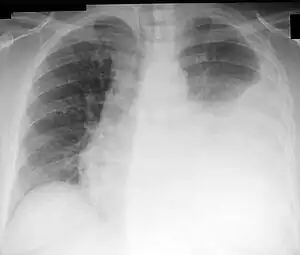 | |
| Chest X-ray showing a left-sided pleural effusion (right side of image) that was than treated with thoracentesis. | |
| Pronunciation |
|
| Specialty | Emergency medicine |
| Indications | Pleural fluid[1] |
| Types | Diagnostic, treatment[1] |
| Steps | 1) Person sitting leaning over table[1] 2) Verify location by ultrasound[1] 3) Clean with chlorhexidine and inject local anesthetic[1] 4) Insert hollow needle just above a rib while aspirating[1] 5) Slowly advance and once fluid reached slide catheter into chest[1] 6) Attach collection container to catheter[1] |
| Complications | Pneumothorax, bleeding, low blood pressure, injury to spleen or liver[1] |
Thoracentesis is a medical procedure to remove fluid from the pleural space for diagnostic or treatment purposes.[1] For diagnosis it is indicated if the cause is unclear and more than 1 centimeter of fluid is present.[1] For treatment it is done to improve shortness of breath.[1] If the fluid reoccurs despite multiple drainages a long term catheter may be placed or pleurodesis may be carried out.[1]
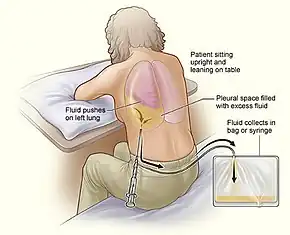
The procedure is typically done with the person sitting and leaning over a table.[1] The location can be verified with ultrasonography at the bedside.[1] The area is cleaned with chlorhexidine and local anesthetic is injected.[1] A hollow needle is than inserted just above a rib at the scapular line.[1] The needle is slowly advanced until fluid is aspirated, after which the catheter is slide into the chest and the needle withdrawn.[1] A collection container may than be attached.[1]
Complications may include pneumothorax, bleeding, low blood pressure, and injury to the spleen or liver.[1] Previously, only about 1.5 liters of fluid would be removed due to the concern of increased risks of reexpansion pulmonary edema with greater volumes.[2] This concern; however, appears to be unfounded and all the fluid present can likely be safely drained.[2] Coughing normally occurs as the lung reexpands.[1]
Thoracentesis was first performed in 1850 by Morrill Wyman and Henry Ingersoll Bowditch.[3] In the United States the procedure can cost about 2,000 USD.[4] The technique had been proposed earlier by Hippocrates in 400 BC.[3] The term is from Greek θώραξ thōrax meaning "chest" and κέντησις kentēsis meaning "puncture".[5]
Medical uses
This procedure is indicated when unexplained fluid accumulates in the chest cavity outside the lung. In more than 90% of cases analysis of pleural fluid yields useful information. If a large amount of fluid is present, then this procedure can also be used therapeutically to remove that fluid and improve comfort and lung function.
Tension pneumothorax requires emergent needle thoracostomy before a chest tube is placed.[6][7]
When cardiopulmonary status is compromised (i.e. when the fluid or air has its repercussions on the function of heart and lungs), due to air (significant pneumothorax), fluid (pleural fluid) or blood (hemothorax) outside the lung, then this procedure is usually replaced with tube thoracostomy.
Technique

The procedure is typically done with the person sitting and leaning over a table.[1] The location can be verified with ultrasonography at the bedside.[1] The area is cleaned with chlorhexidine and local anesthetic is injected.[1] A hollow needle is than inserted just above a rib at the scapular line.[1] The needle is slowly advanced until fluid is aspirated, after which the catheter is slide into the chest and the needle withdrawn.[1] A collection container may than be attached.[1]
A skin marker or back of a pen can be used to mark the spot identified by ultrasound, such that it will not be washed off with the cleaning solution.[1]
Complications
Major complications are pneumothorax (3–30%), hemopneumothorax, hemorrhage, hypotension (low blood pressure due to a vasovagal response) and reexpansion pulmonary edema.
Minor complications include a dry tap (no fluid return), subcutaneous hematoma or seroma, anxiety, shortness of breath and cough (after removing large volume of fluid).
Ultrasound guidance reduces complications.[9][10][11]
Contraindications
An uncooperative person or a coagulation disorder that cannot be corrected are relative contraindications.[12] Routine measurement of coagulation profiles is generally not indicated, however; when performed by an experienced operator "hemorrhagic complications are infrequent after ultrasound-guided thoracentesis, and attempting to correct an abnormal INR or platelet level before the procedure is unlikely to confer any benefit."[13]
Relative contraindications include cases in which the site of insertion has known bullous emphysema, use of positive end-expiratory pressure (PEEP, see mechanical ventilation) and only one functioning lung (due to diminished reserve).
Follow-up imaging
A chest X-ray has traditionally been performed to assess for pneumothorax following the procedure; however, it may not be necessary to do so in asymptomatic, non-ventilated persons given the widespread use of ultrasound to guide the procedure.[14]
Fluid analysis
Several diagnostic tools are available to determine the cause of pleural fluid. The most common causes are cancer, congestive heart failure, pneumonia, and recent surgery. In countries where tuberculosis is common, this is also a common cause.
Transudate versus exudate
First the fluid is either transudate or exudate.
An exudate is defined as pleural fluid to serum total protein ratio of more than 0.5, pleural fluid to serum LDH ratio > 0.6, and absolute pleural fluid LDH > 200 IU or > 2/3 of the normal.
An exudate is defined as pleural fluid that filters from the circulatory system into lesions or areas of inflammation. Its composition varies but generally includes water and the dissolved solutes of the main circulatory fluid such as blood. In the case of blood it will contain some or all plasma proteins, white blood cells, platelets and (in the case of local vascular damage) red blood cells.
Exudate
- Blood
- Infection
- Inflammation
- Malignancy
- Iatrogenic
- Connective tissue disease
- Endocrine disorders
- Lymphatic disorders vs Constrictive pericarditis
Transudate
- Congestive heart failure
- Nephrotic syndrome
- Hypoalbuminemia
- Cirrhosis
- Atelectasis
- Trapped lung
- Peritoneal dialysis
- Superior vena cava obstruction
Amylase
A high amylase level (twice the serum level or the absolute value is greater than 160 Somogy units) in the pleural fluid is indicative of either acute or chronic pancreatitis, pancreatic pseudocyst that has dissected or ruptured into the pleural space, cancer or esophageal rupture.
Glucose
Glucose is considered low if pleural fluid value is less than 50% of normal serum value. The differential diagnosis for this is:
- rheumatoid effusion. The levels are characteristically low (<15 mg/dL).
- lupus effusion
- bacterial empyema
- malignancy
- tuberculosis
- esophageal rupture (Boerhaave syndrome)
pH
Normal pleural fluid pH is approximately 7.60. A pleural fluid pH below 7.30 with normal arterial blood pH has the same differential diagnosis as low pleural fluid glucose.
Triglyceride and cholesterol
Chylothorax (fluid from lymph vessels leaking into the pleural cavity) may be identified by determining triglyceride and cholesterol levels, which are relatively high in lymph. A triglyceride level over 110 mg/dl and the presence of chylomicrons indicate a chylous effusion. The appearance is generally milky but can be serous.
The main cause for chylothorax is rupture of the thoracic duct, most frequently as a result of trauma or malignancy (such as lymphoma).
Cell count and differential
The number of white blood cells can give an indication of infection. The specific subtypes can also give clues as to the type on infection. The amount of red blood cells are an obvious sign of bleeding.
Cultures and stains
If the effusion is caused by infection, microbiological culture may yield the infectious organism responsible for the infection, sometimes before other cultures (e.g. blood cultures and sputum cultures) become positive. A Gram stain may give a rough indication of the causative organism. A Ziehl–Neelsen stain may identify tuberculosis or other mycobacterial diseases.
Cytology
Cytology is an important tool in identifying effusions due to malignancy. The most common causes for pleural fluid are lung cancer, metastasis from elsewhere and pleural mesothelioma. The latter often presents with an effusion. Normal cytology results do not reliably rule out malignancy, but make the diagnosis more unlikely.
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 "How To Do Thoracentesis - Pulmonary Disorders". Merck Manuals Professional Edition. Archived from the original on 16 June 2022. Retrieved 8 August 2022.
- 1 2 Farkas, Josh (12 August 2019). "PulmCrit- Large volume thora: Can we drain 'em dry?". EMCrit Project. Archived from the original on 29 January 2022. Retrieved 8 August 2022.
- 1 2 Sebastian, Anton (6 February 2018). A Dictionary of the History of Medicine. Routledge. p. PT1366. ISBN 978-1-351-46999-9. Archived from the original on 10 August 2022. Retrieved 8 August 2022.
- ↑ "Thoracentesis". www.mdsave.com. Archived from the original on 5 August 2020. Retrieved 9 August 2022.
- ↑ Haubrich, William S. (November 2003). Medical Meanings: A Glossary of Word Origins. ACP Press. p. 240. ISBN 978-1-930513-49-5. Archived from the original on 2022-08-10. Retrieved 2022-08-08.
- ↑ Harcke, HT; Mabry, RL; Mazuchowski, EL (2013). "Needle thoracentesis decompression: observations from postmortem computed tomography and autopsy". Journal of Special Operations Medicine. 13 (4): 53–8. PMID 24227562.
- ↑ Ball, Chad G.; Wyrzykowski, Amy D.; Kirkpatrick, Andrew W.; Dente, Christopher J.; Nicholas, Jeffrey M.; Salomone, Jeffrey P.; Rozycki, Grace S.; Kortbeek, John B.; Feliciano, David V. (2010). "Thoracic needle decompression for tension pneumothorax: clinical correlation with catheter length". Canadian Journal of Surgery. 53 (3): 184–188. PMC 2878990. PMID 20507791.
- ↑ de Menezes Lyra R (1997). "A modified outer cannula can help thoracentesis after pleural biopsy". Chest. 112 (1): 296. doi:10.1378/chest.112.1.296. PMID 9228404.
- ↑ Gordon, Craig E.; Feller-Kopman, D; Balk, EM; Smetana, GW (22 February 2010). "Pneumothorax Following Thoracentesis". Archives of Internal Medicine. 170 (4): 332–9. doi:10.1001/archinternmed.2009.548. PMID 20177035.
- ↑ Feller-Kopman, David (July 2007). "Therapeutic thoracentesis: the role of ultrasound and pleural manometry". Current Opinion in Pulmonary Medicine. 13 (4): 312–318. doi:10.1097/MCP.0b013e3281214492. PMID 17534178.
- ↑ Daniels, Craig E; Ryu, Jay H (July 2011). "Improving the safety of thoracentesis". Current Opinion in Pulmonary Medicine. 17 (4): 232–236. doi:10.1097/MCP.0b013e328345160b. PMID 21346571.
- ↑ "Thoracentesis (section)". Merck Manual. Merck Manual. Archived from the original on 7 November 2014. Retrieved 7 November 2014.
- ↑ Hibbert, Rebecca M.; Atwell, Thomas D.; Lekah, Alexander; Patel, Maitray D.; Carter, Rickey E.; McDonald, Jennifer S.; Rabatin, Jeffrey T. (August 2013). "Safety of Ultrasound-Guided Thoracentesis in Patients With Abnormal Preprocedural Coagulation Parameters". Chest. 144 (2): 456–463. doi:10.1378/chest.12-2374. PMID 23493971.
- ↑ Petersen, William G.; Zimmerman, Robert (April 2000). "Limited Utility of Chest Radiograph After Thoracentesis". Chest. 117 (4): 1038–1042. doi:10.1378/chest.117.4.1038. PMID 10767236.
External links
| Classification | |
|---|---|
| External resources |
- A photo gallery of thoracentesis showing the procedure step-by-step Archived 2022-04-07 at the Wayback Machine. V. Dimov, B. Altaqi, Clinical Notes, 2005.