Hereditary amyloidosis transthyretin-related
| Hereditary amyloidosis transthyretin-related | |
|---|---|
| Other names: Corino de Andrade's disease; amyloidosis hereditary transthyretin-related | |
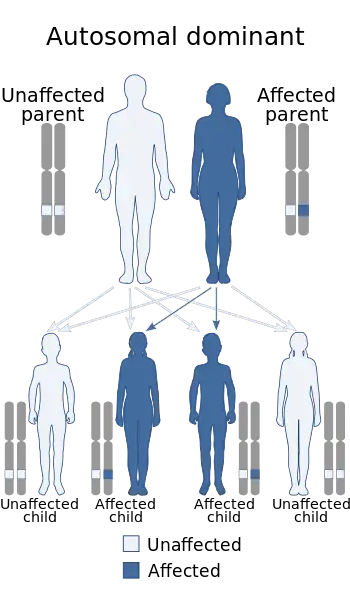 | |
| Hereditary amyloidosis transthyretin-related has an autosomal dominant pattern of inheritance. | |
| Specialty | Neurology |
| Symptoms | Numbness of the limbs, carpal tunnel syndrome, autonomic dysfunction, cardiomyopathy[1][2] |
| Complications | Severe diarrhea, weight loss, low blood pressure with standing[2] |
| Usual onset | 40 to 65 years[3] |
| Types | Familial amyloid polyneuropathy, cardiac amyloidosis, leptomeningeal amyloidosis[2] |
| Causes | Genetic (autosomal dominant)[2] |
| Diagnostic method | Based on symptoms, biopsy, genetic testing[1] |
| Treatment | Liver transplantation[3] |
| Medication | Patisiran, inotersen, vutrisiran, tafamidis[3][4] |
| Frequency | Rare[1] |
| Deaths | Life expectancy 5 to 15 yr post onset[2] |
Hereditary amyloidosis transthyretin-related (hATTR) is a condition that can result in numbness of the limbs, carpal tunnel syndrome, autonomic dysfunction, and cardiomyopathy.[1][2] As the disease progresses severe diarrhea, weight loss, and low blood pressure with standing may occur.[2]
It is due to a autosomal dominant genetic mutation which is inherited from a person's parents or newly occurs during early development.[2][3] Various mutations may be involved.[2] The underlying mechanism involves build-up of the protein amyloid in body tissues.[1] Diagnosis is based on symptoms, biopsy, and genetic testing.[1] It is a type of amyloidosis.[3]
Treatments may include liver transplantation in early disease.[3] The medications patisiran, inotersen, or vutrisiran may be used for nerve problems while tafamidis may be used for heart problems.[3][4] Death generally occurs around 10 years after symptom onset.[2]
Familial amyloid polyneuropathy occurs in about 4% of African Americans and 1 in 100,000 white people.[3] Onset of symptoms is usually in those 40 to 65 years old.[3] It was first described by Portuguese neurologist Mário Corino da Costa Andrade, in 1952.[2][5]
Signs and symptoms
Onset of symptoms is usually between 40 and 65 years of age,[3] with pain, paresthesia, muscular weakness and autonomic dysfunction. In its late state, the kidneys and the heart are affected. FAP is characterized by the systemic deposition of amyloidogenic variants of the transthyretin protein, especially in the peripheral nervous system, causing a progressive sensory and motor polyneuropathy.
Cause
FAP is caused by a mutation of the TTR gene, located on human chromosome 18q12.1-11.2.[6] A replacement of valine by methionine at position 30 (TTR V30M) is the mutation most commonly found in FAP.[7] The transthyretin protein is a tetramer. The tetramer has to dissociate into misfolded monomers to aggregate into a variety of structures including amyloid fibrils. Because most patients are heterozygotes, they deposit both mutant and wild type TTR subnits.
FAP is inherited in an autosomal dominant manner.[8] This means that the defective gene responsible for the disorder is located on an autosome (chromosome 18 is an autosome), and only one copy of the defective gene is sufficient to cause the disorder, when inherited from a parent who has the disorder.
Diagnosis
Clinical suspicion is raised on the basis of a family history of neuropathy and physical exam showing signs of neuropathy. Diagnosis can be made using genetic testing to identify mutations in the TTR gene, but may include other corroborative investigation.[9] Nerve conduction testing typically shows an axonal polyneuropathy, with sensory involvement greater than motor. Superimposed mononeuropathies may also be evident, such as a median mononeuropathy at the wrist (carpal tunnel syndrome). Electromyography (EMG) may show evidence of chronic denervation and reinnervation. Autonomic testing, including quantitative sweat testing, can reveal involvement of the autonomic nervous system.[10] Occasionally, biopsy of skin, nerve, or muscle may be performed, which can show signs of denervation and amyloid deposition with response to anti-TTR antibodies.[11] Additional testing should be performed to identify involvement of the heart or kidneys.[9]
Sudomotor function through electrochemical skin conductance may provide a measure of subclinical autonomic involvement.[12][13]
It is a type of amyloidosis, distinct from senile systemic amyloidosis (SSA), which is not inherited, and which was determined to be the primary cause of death for 70% of supercentenarians.[14]
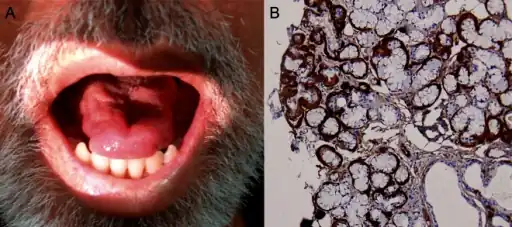 a)Tongue atrophy due to transthyretin-related familial amyloid polyneuropathy(synonym [15]) b)minor salivary gland biopsy
a)Tongue atrophy due to transthyretin-related familial amyloid polyneuropathy(synonym [15]) b)minor salivary gland biopsy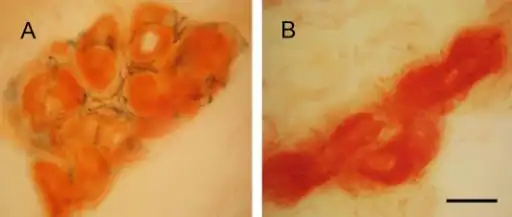 Sweat gland innervations in familial amyloid polyneuropathy a) control b)sweat gland innervation markedly reduced in FAP
Sweat gland innervations in familial amyloid polyneuropathy a) control b)sweat gland innervation markedly reduced in FAP
Treatments
The medication tafamidis has been approved for the treatment of transthyretin transthyretin-mediated amyloidosis in Europe and the United States.[16][17] Studies have found that it delays neurological problems when started early.[16][18]
In 2018, patisiran was approved in the United States, an siRNA-based treatment, at an expected cost of $450,000 per year.[19]
Prognosis
In the absence of a liver transplant, FAP is invariably fatal, usually within a decade. The disadvantage of liver transplantation is that approximately 10% of the subjects die from the procedure or complications resulting from the procedure, which is a form of gene therapy wherein the liver expressing wild type and mutant TTR is replaced by a liver only expressing wild type TTR. Moreover, transplanted patients must take immune suppressants (medications) for the remainder of their life, which can lead to additional complications. In late 2011, the European Medicines Agency approved the transthyretin kinetic stabilizer Tafamidis or Vyndaqel discovered by Jeffery W. Kelly and developed by FoldRx pharmaceuticals (acquired by Pfizer in 2010) for the treatment of FAP based on clinical trial data. Tafamidis (20 mg once daily) slowed the progression of FAP over a 36-month period and importantly reversed the weight loss and muscle wasting associated with disease progression.
Epidemiology
This disease is relatively common in Portuguese locations Póvoa de Varzim and Vila do Conde (Caxinas), with more than 1000 affected people, coming from about 500 families, where 70% of the people develop the illness. ll the analysed Portuguese families presented the same haplotype (haplotype I) associated with the Met 30 mutation. In northern Sweden, more specifically Skellefteå (it is locally called "Skelleftesjukan", the Skellefteå disease), 1.5% of the population has the mutated gene. There are many other populations in the world who exhibit the illness after having developed it independently.
Research
In August 2021 six patients with hereditary ATTR amyloidosis with polyneuropathy were given doses of NTLA-2001, based on a CRISPR gene editing system. Researchers reported mild adverse events and decreases in serum misfolded transthyretin protein concentrations through targeted knockout.[20]
References
- 1 2 3 4 5 6 "Familial transthyretin amyloidosis - About the Disease - Genetic and Rare Diseases Information Center". rarediseases.info.nih.gov. Archived from the original on 15 November 2022. Retrieved 13 December 2022.
- 1 2 3 4 5 6 7 8 9 10 11 "#105210 - AMYLOIDOSIS, HEREDITARY, TRANSTHYRETIN-RELATED". omim.org. Archived from the original on 6 December 2022. Retrieved 13 December 2022.
- 1 2 3 4 5 6 7 8 9 10 "Amyloidosis". NORD (National Organization for Rare Disorders). Archived from the original on 18 August 2022. Retrieved 12 December 2022.
- 1 2 "DailyMed - AMVUTTRA- vutrisiran injection". dailymed.nlm.nih.gov. Archived from the original on 3 July 2022. Retrieved 13 December 2022.
- ↑ Andrade C (September 1952). "A peculiar form of peripheral neuropathy; familiar atypical generalized amyloidosis with special involvement of the peripheral nerves". Brain. 75 (3): 408–27. doi:10.1093/brain/75.3.408. PMID 12978172.
- ↑ Online Mendelian Inheritance in Man (OMIM): Transthyretin (TTR) - 176300
- ↑ Online Mendelian Inheritance in Man (OMIM): Amyloidosis, hereditary, transthyretin-related - 105210
- ↑ Ando Y, Ueda M (May 2008). "Novel methods for detecting amyloidogenic proteins in transthyretin related amyloidosis". Frontiers in Bioscience. 13 (13): 5548–58. doi:10.2741/3098. PMID 18508604.
- 1 2 Adams, David; Ando, Yukio; Beirão, João Melo; Coelho, Teresa; Gertz, Morie A.; Gillmore, Julian D.; Hawkins, Philip N.; Lousada, Isabelle; Suhr, Ole B.; Merlini, Giampaolo (6 January 2020). "Expert consensus recommendations to improve diagnosis of ATTR amyloidosis with polyneuropathy". Journal of Neurology. 268 (6): 2109–2122. doi:10.1007/s00415-019-09688-0. PMC 8179912. PMID 31907599.
- ↑ Kim, Dong Hwee; Zeldenrust, Steven R.; Low, Phillip A.; Dyck, Peter J. (September 2009). "Quantitative sensation and autonomic test abnormalities in transthyretin amyloidosis polyneuropathy". Muscle & Nerve. 40 (3): 363–370. doi:10.1002/mus.21332. PMC 2735590. PMID 19618439.
- ↑ Shin, Susan C.; Robinson-Papp, Jessica (November 2012). "Amyloid Neuropathies". The Mount Sinai Journal of Medicine, New York. 79 (6): 733–748. doi:10.1002/msj.21352. ISSN 0027-2507. PMC 3531896. PMID 23239211.
- ↑ Lefaucheur, J. P.; Zouari, H. G.; Gorram, F.; Nordine, T.; Damy, T.; & Planté-Bordeneuve, V. (2018). "The value of electrochemical skin conductance measurement using Sudoscan® in the assessment of patients with familial amyloid polyneuropathy". Clinical Neurophysiology. 129 (8): 1565–1569. doi:10.1016/j.clinph.2018.05.005. PMID 29883834. S2CID 47011006
- ↑ Castro, J.; Costa, J.; de Castro, I.; & Conceição, I. (2018). "Electrochemical skin conductance in hereditary amyloidosis related to transthyretin V30M–a promising tool to assess treatment efficacy?". Amyloid. 25 (4): 267–268. doi:10.1080/13506129.2018.1545639. PMID 30773060. S2CID 73476147.
- ↑ Coles LS, Young RD (May 2012). "Supercentenarians and transthyretin amyloidosis: the next frontier of human life extension". Preventive Medicine. 54 Suppl (Suppl): S9–11. doi:10.1016/j.ypmed.2012.03.003. PMID 22579241.
- ↑ "Amyloidogenic transthyretin amyloidosis (Concept Id: C2751492) - MedGen - NCBI". www.ncbi.nlm.nih.gov. Archived from the original on 2023-03-06. Retrieved 2023-09-08.
- 1 2 Adams, D; Cauquil, C; Labeyrie, C (October 2017). "Familial amyloid polyneuropathy". Current Opinion in Neurology. 30 (5): 481–489. doi:10.1097/WCO.0000000000000476. PMID 28678039. S2CID 4968350.
- ↑ "Tafamidis Monograph for Professionals". Drugs.com. Archived from the original on 9 July 2021. Retrieved 13 December 2022.
- ↑ Scott LJ (August 2014). "Tafamidis: a review of its use in familial amyloid polyneuropathy". Drugs. 74 (12): 1371–8. doi:10.1007/s40265-014-0260-2. PMID 25022953. S2CID 24612955.
- ↑ "Rare-Disease Treatment From Alnylam to Cost $450,000 a Year". www.bloomberg.com. Archived from the original on 2019-11-14. Retrieved 11 August 2018.
- ↑ Gillmore, Julian D. (August 5, 2021). "CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis". The New England Journal of Medicine. 385 (6): 493–502. doi:10.1056/NEJMoa2107454. PMID 34215024. S2CID 235722446. Archived from the original on May 22, 2019. Retrieved March 19, 2022.
External links
| Classification | |
|---|---|
| External resources |
- GeneReviews/NIH/NCBI/UW entry on Familial Transthyretin Amyloidosis Archived 2022-01-20 at the Wayback Machine