Trypanosoma cruzi
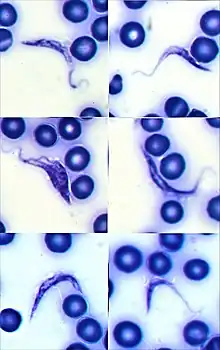
Trypanosoma cruzi in human blood Giemsa stain. They are typically seen as a C-shape and have a more pronounced kinetoplast compared to other species.
Scientific Classification
Domain: Eukaryota
Phylum: Euglenozoa
Class: Kinetoplastea
Order: Trypanosomatida
Family:Trypanosomatidae
Genus: Trypanosoma
Species: T. cruzi
Trypanosoma cruzi is a species of parasitic euglenoids. Among the protozoa, the trypanosomes characteristically bore tissue in another organism and feed on blood (primarily) and also lymph. This behaviour causes disease or the likelihood of disease that varies with the organism: Chagas disease in humans, dourine in horses.[1][2]
Parasites need a host body and the haematophagous insect triatomine is the major vector in accord with a mechanism of infection. The triatomine likes the nests of vertebrate animals for shelter, where it bites and sucks blood for food. Individual triatomines infected with protozoa from other contact with animals transmit trypanosomes when the triatomine deposits its faeces on the host's skin surface and then bites. Penetration of the infected faeces is further facilitated by the scratching of the bite area by the human or animal host.[1][2][3]
General characteristics
Trypanosoma cruzi, is a parasitic protozoan that causes American trypanosomiasis or Chagas. When T. cruzi enters a host (mammal) this causes the activation of signaling cascades and mobilization (such as Ca2+).Currently, six distinct lineages of T. cruzi exist[4][5][6]:
- TcI
- TcII
- TcIII
- TcIV
- TcV
- TcVI
.png.webp)
Mechanism
Trypanosomiasis in humans progresses with the development of the trypanosome into a trypomastigote in the blood and into an amastigote in tissues. The acute form of trypanosomiasis is usually unnoticed, although it may manifest itself as a localized swelling at the site of entry. The chronic form may develop 30 to 40 years after infection and affect internal organs.[7][8][9]
Acute cases are treated with nifurtimox and benznidazole, but no effective therapy for chronic cases is currently known.[10]
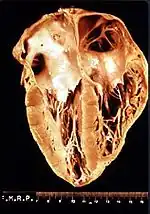
Cardiac manifestations
Researchers of Chagas’ disease have demonstrated several processes that occur with all cardiomyopathies. The first event is an inflammatory response. Following inflammation, cellular damage occurs. Finally, in the body's attempt to recover from the cellular damage, fibrosis begins in the cardiac tissue.[11]
Another cardiomyopathy found in nearly all cases of chronic Chagas’ disease is thromboembolic syndrome. Thromboembolism describes thrombosis, the formation of a clot, and its main complication is embolism, the carrying of a clot to a distal section of a vessel and causing blockage there. This occurrence contributes to the death of a patient by four means: arrhythmias, stasis secondary to cardiac dilation, mural endocarditis, and cardiac fibrosis. These thrombi also affect other organs such as the brain, spleen and kidney.[12]
Myocardial biochemical response
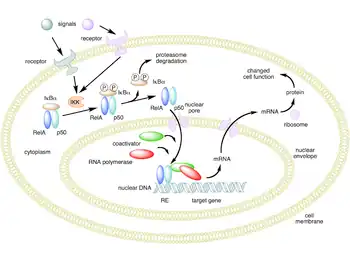
Subcellular findings in murine studies with induced T. cruzi infection revealed that the chronic state is associated with the persistent elevation of phosphorylated (activated) extracellular-signal-regulated kinase (ERK), AP-1, and NF-κB. Also, the mitotic regulator for G1 progression, cyclin D1 was found to be activated. Although there was no increase in any isoform of ERK, there was an increased concentration of phosphorylated ERK in mice infected with T. cruzi. It was found that within seven days the concentration of AP-1 was significantly higher in T. cruzi–infected mice when compared to the control. Elevated levels of NF-κB have also been found in myocardial tissue, with the highest concentrations being found in the vasculature. It was indicated through Western blot that cyclin D1 was upregulated from day 1 to day 60 post-infection. It was also indicated through immunohistochemical analysis that the areas that produced the most cyclin D1 were the vasculature and interstitial regions of the heart.[13]
Rhythm abnormalities
Conduction abnormalities are also associated with T. cruzi. At the base of these conduction abnormalities is a depopulation of parasympathetic neuronal endings on the heart. Without proper parasympathetic innervations, one could expect to find not only chronotropic but also inotropic abnormalities. It is true that all inflammatory and non-inflammatory heart disease may display forms of parasympathetic denervation; this denervation presents in a descriptive fashion in Chagas’ disease. It has also been indicated that the loss of parasympathetic innervations can lead to sudden death due to a severe cardiac failure that occurs during the acute stage of infection.[14]
Another conduction abnormality presented with chronic Chagas’ disease is a change in ventricular repolarization, which is represented on an electrocardiogram as the T-wave. This change in repolarization inhibits the heart from relaxing and properly entering diastole. Changes in the ventricular repolarization in Chagas’ disease are likely due to myocardial ischemia. This ischemia can also lead to fibrillation. This sign is usually observed in chronic Chagas’ disease and is considered a minor electromyocardiopathy.[15]
Epicardial lesions
Villous plaque is characterized by exophytic epicardial thickening, meaning that the growth occurs at the border of the epicardium and not the center of mass. Unlike milk spots and chagasic rosary, inflammatory cells and vasculature are present in villous plaque. Since villous plaque contains inflammatory cells it is reasonable to suspect that these lesions are more recently formed than milk spots or chagasic rosary.[16]
Molecular mechanism
T. cruzi does not produce prostaglandins itself. Instead Pinge-Filho et al 1999 finds that the parasite induces mice to overproduce 2-series prostaglandins themselves.[17] These PG2s are immunosuppressive and so aid in immune evasion.[17]
Imipramines are trypanocidal.[17] Doyle & Weinbach 1989 find imipramine and various of its derivatives – 3-Chlorimipramine, 2-Nitroimipramine, and 2-Nitrodesmethylimipramine – are trypanocidal in vitro.[17] They find 2-Nitrodesmethylimipramine is the most effective among them.[17]
Life cycle
.jpg.webp)
The Trypanosoma cruzi life cycle starts in an animal reservoir, usually mammals, wild or domestic, including humans. A triatomine bug serves as the vector. While taking a blood meal, it ingests T. cruzi. In the triatomine bug (Triatoma infestans) the parasite goes into the epimastigote stage, making it possible to reproduce. After reproducing through binary fission, the epimastigotes move onto the rectal cell wall, where they become infectious. Infectious T. cruzi are called metacyclic trypomastigotes. When the triatomine bug subsequently takes a blood meal from a host, it defecates - its waste containing T. cruzi propagation stages. This is the parasite's only transmission route. As a result Trumper and Gorla 1991 find transmission success centers around the triatomine's defecation behaviors.[18][19][20] The trypomastigotes are in the feces and are capable of swimming into the host's cells using flagella, a characteristic swimming tail dominant in the Euglenoid class of protists.[21]
The trypomastigotes enter the host through the bite wound or by crossing mucous membranes. The host cells contain macromolecules such as laminin, thrombospondin, heparin sulphate, and fibronectin that cover their surface.[22] These macromolecules are essential for adhesion between parasite and host and for the process of host invasion by the parasite. The trypomastigotes must cross a network of proteins that line the exterior of the host cells in order to make contact and invade the host cells. The molecules and proteins on the cytoskeleton of the cell also bind to the surface of the parasite and initiate host invasion.[22]
Genetic exchange
Genetic exchange has been identified among field populations of T. cruzi.[23] This process appears to involve genetic recombination as well as a meiotic mechanism. Despite the capability for sexual reproduction, natural populations of T. cruzi exhibit clonal population structures. It appears that frequent sexual reproduction events occur primarily between close relatives resulting in an apparent clonal population structure.[24]
Disease
.jpg.webp) Trypanosoma cruzi amistigotes
Trypanosoma cruzi amistigotes.jpg.webp) Trypanosoma cruzi
Trypanosoma cruzi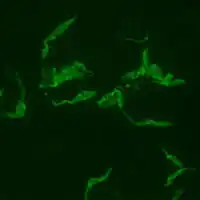 Positive IFA result with T. cruzi antigen
Positive IFA result with T. cruzi antigen
The incubation period is five to fourteen days after a host comes in contact with feces. Chagas disease undergoes two phases, which are the acute and the chronic phase. The acute phase can last from two weeks to two months but can go unnoticed because symptoms are minor and short-lived. Symptoms of the acute phase include swelling, fever, fatigue, and diarrhea. The chronic phase causes digestive problems, constipation, heart failure, and pain in the abdomen. Should the individual have HIV then an opportunistic infection as this would "represent reactivation and not acute infection with T. cruzi"'.[25][26] [27][28]
The presence of T. cruzi is diagnostic of Chagas disease. During the acute phase of infection, it can be detected by microscopic examination of fresh anticoagulated blood, or its buffy coat, for motile parasites; or by preparation of thin and thick blood smears stained with Giemsa, for direct visualization of parasites.[29][30] On microscopic examination, T. cruzi trypomastigotes have a slender body, often in the shape of an S or U, with a flagellum connected to the body by an undulating membrane.[31]
Alternatively, T. cruzi DNA can be detected by polymerase chain reaction (PCR). In acute and congenital Chagas disease, PCR is more sensitive than microscopy,[32] and it is more reliable than antibody-based tests for the diagnosis of congenital disease because it is not affected by transfer of antibodies against T. cruzi from a mother to her baby (passive immunity).[33]
No vaccines are available. The most used method for epidemiological management and disease prevention resides within vector control,[34] mainly by the use of insecticides and taking preventative measures such as applying bug repellent on the skin, wearing protective clothing, and staying in higher quality hotels when traveling. Investing in quality housing would be ideal to decrease risk of contracting this disease.[35]
Epidemiology
T. cruzi transmission has been documented in the Southwestern U.S., and warming trends may allow vector species to move north. U.S. domestic and wild animals are reservoirs for T. cruzi. Triatomine species in the southern U.S. have taken human blood meals, but because triatomines do not favor typical U.S. housing, risk to the U.S. population is very low.[36]
.svg.png.webp)
Chagas' disease's geographical occurrence happens worldwide but high-risk individuals include those who don't have access to proper housing. Its reservoir is in wild animals but its vector is a kissing bug. This is a contagious disease and can be transmitted through a number of ways: congenital transmission, blood transfusion, organ transplantation, consumption of uncooked food that has been contaminated with feces.[37][38]
Over 130 species can transmit this parasite[39]Six taxonomic subunits are recognised.[40]
In 2017, an estimated 6.2 million people worldwide had Chagas disease, with approximately 162,000 new infections and 7,900 deaths each year.[41][42] Chagas disease results in the loss of over 800,000 disability-adjusted life years each year.[8]
Chagas is endemic to 21 countries in continental Latin America: Argentina, Belize, Bolivia, Brazil, Chile, Colombia, Costa Rica, Ecuador, El Salvador, French Guiana, Guatemala, Guyana, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Suriname, Uruguay, and Venezuela.[43][8] The endemic area ranges from the southern United States to northern Chile and Argentina, with Bolivia (6.1%), Argentina (3.6%), and Paraguay (2.1%) exhibiting the highest prevalence of the disease.[8] In endemic areas, due largely to vector control efforts and screening of blood donations, annual infections and deaths have fallen by 67% and more than 73% respectively from their peaks in the 1980s to 2010.[8][44] Transmission by insect vector and blood transfusion has been completely interrupted in Uruguay (1997), Chile (1999), and Brazil (2006),[44] and in Argentina, vectorial transmission has been interrupted in 13 of the 19 endemic provinces.[45] During Venezuela's humanitarian crisis, vectorial transmission has begun occurring in areas where it had previously been interrupted and Chagas disease seroprevalence rates have increased.[46] Transmission rates have also risen in the Gran Chaco region due to insecticide resistance and in the Amazon basin due to oral transmission.[8]
While the rate of vector-transmitted Chagas disease has declined throughout most of Latin America, the rate of orally transmitted disease has risen, possibly due to increasing urbanization and deforestation bringing people into closer contact with triatomines and altering the distribution of triatomine species.[47][48] Orally transmitted Chagas disease is of particular concern in Venezuela, where 16 outbreaks have been recorded between 2007 and 2018.[49]
Chagas exists in two different ecological zones: In the Southern Cone region, the main vector lives in and around human homes. In Central America and Mexico, the main vector species lives both inside dwellings and in uninhabited areas. In both zones, Chagas occurs almost exclusively in rural areas, where T. cruzi also circulates in wild and domestic animals.[50] T. cruzi commonly infects more than 100 species of mammals across Latin America including opossums, armadillos, marmosets, bats, and various rodents, all of which can be infected by the vectors or orally by eating triatomine bugs and other infected animals.[51]
See also
References
- 1 2 de Lana, M.; de Menezes Machado, E. M. (1 January 2017). "16 - Biology of Trypanosoma cruzi and biological diversity". American Trypanosomiasis Chagas Disease (Second Edition). Elsevier. pp. 345–369. ISBN 978-0-12-801029-7. Archived from the original on 22 July 2022. Retrieved 20 November 2022.
- 1 2 Souza, Wanderley De (18 December 2019). Biology of Trypanosoma cruzi. BoD – Books on Demand. p. 3. ISBN 978-1-83968-203-2. Archived from the original on 22 November 2022. Retrieved 22 November 2022.
- ↑ Delgado, María-Jesús Pinazo; Gascón, Joaquim (20 July 2020). Chagas Disease: A Neglected Tropical Disease. Springer Nature. p. 38. ISBN 978-3-030-44054-1. Archived from the original on 25 November 2022. Retrieved 25 November 2022.
- ↑ Yoshida, Nobuko; Cortez, Mauro (2008). "Trypanosoma cruzi: parasite and host cell signaling during the invasion process". Sub-Cellular Biochemistry. 47: 82–91. doi:10.1007/978-0-387-78267-6_6. ISSN 0306-0225. Archived from the original on 21 June 2022. Retrieved 24 November 2022.
- ↑ "CDC - DPDx - American Trypanosomiasis". www.cdc.gov. 14 June 2021. Archived from the original on 28 October 2022. Retrieved 18 November 2022.
- ↑ de Oliveira, Maykon Tavares; Machado de Assis, Girley Francisco; Oliveira e Silva, Jaquelline Carla Valamiel; Machado, Evandro Marques Menezes; da Silva, Glenda Nicioli; Veloso, Vanja Maria; Macedo, Andrea Mara; Martins, Helen Rodrigues; de Lana, Marta (31 October 2015). "Trypanosoma cruzi Discret Typing Units (TcII and TcVI) in samples of patients from two municipalities of the Jequitinhonha Valley, MG, Brazil, using two molecular typing strategies". Parasites & Vectors. 8 (1): 568. doi:10.1186/s13071-015-1161-2. ISSN 1756-3305. Archived from the original on 19 November 2022. Retrieved 18 November 2022.
- ↑ Altcheh, Jaime Marcelo; Freilij, Hector (9 September 2019). Chagas Disease: A Clinical Approach. Springer Nature. p. 244. ISBN 978-3-030-00054-7. Archived from the original on 19 November 2022. Retrieved 19 November 2022.
- 1 2 3 4 5 6 Pérez-Molina JA, Molina I (2018). "Chagas disease". The Lancet. 391 (10115): 82–94. doi:10.1016/S0140-6736(17)31612-4. ISSN 0140-6736.
- ↑ Podlipaev, Sergei (1 May 2001). "The more insect trypanosomatids under study-the more diverse Trypanosomatidae appears". International Journal for Parasitology. 31 (5): 648–652. doi:10.1016/S0020-7519(01)00139-4. ISSN 0020-7519. Retrieved 29 November 2022.
- ↑ Prevention, CDC-Centers for Disease Control and (11 April 2022). "CDC - Chagas Disease - Resources for Health Professionals - Antiparasitic Treatment". www.cdc.gov. Archived from the original on 6 November 2016. Retrieved 22 November 2022.
- ↑ Leiby, David A.; Herron Jr, Ross M.; Read, Elizabeth J.; Lenes, Bruce A.; Stumpf, Robert J. (2002). "Trypanosoma cruzi in Los Angeles and Miami blood donors: Impact of evolving donor demographics on seroprevalence and implications for transfusion transmission". Transfusion. 42 (5): 549–55. doi:10.1046/j.1537-2995.2002.00077.x. PMID 12084162. S2CID 11997057.
- ↑ Marin-Neto, Jose Antonio; Cunha-Neto, Edécio; MacIel, Benedito C.; Simões, Marcus V. (2007). "Pathogenesis of Chronic Chagas Heart Disease". Circulation. 115 (9): 1109–23. doi:10.1161/CIRCULATIONAHA.106.624296. PMID 17339569.
- ↑ Huang, Huan; Petkova, Stefka B.; Cohen, Alex W.; Bouzahzah, Boumediene; Chan, John; Zhou, Jian-nian; Factor, Stephen M.; Weiss, Louis M.; Krishnamachary, Mohan; Mukherjee, Shankar; Wittner, Murray; Kitsis, Richard N.; Pestell, Richard G.; Lisanti, Michael P.; Albanese, Chris; Tanowitz, Herbert B. (2003). "Activation of Transcription Factors AP-1 and NF- B in Murine Chagasic Myocarditis". Infection and Immunity. 71 (5): 2859–67. doi:10.1128/IAI.71.5.2859-2867.2003. PMC 153290. PMID 12704159.
- ↑ Baroldi, Giorgio; Oliveira, Samuel J.M; Silver, Malcolm D (1997). "Sudden and unexpected death in clinically 'silent' Chagas' disease. A hypothesis". International Journal of Cardiology. 58 (3): 263–8. doi:10.1016/S0167-5273(96)02878-1. PMID 9076552.
- ↑ Valente, Ney; Pimenta, João; Paola, Angelo Amato Vincenzo de (2006). "Estudos eletrofisiológicos seriados do sistema éxcito-condutor do coração de pacientes com cardiopatia chagásica crônica" [Serial electrophysiological studies of the heart's excito-conductor system in patients with chronic chagasic cardiopathy]. Arquivos Brasileiros de Cardiologia (in português). 86 (1): 19–25. doi:10.1590/S0066-782X2006000100004. PMID 16491205.
- ↑ Benvenuti, Luiz Alberto; Gutierrez, Paulo Sampaio (2007). "Lesões epicárdicas na cardiopatia chagásica são reflexo de processo inflamatório" [Epicardial lesions in Chagas' heart disease reflect an inflammatory process]. Arquivos Brasileiros de Cardiologia (in português). 88 (4): 496–8. doi:10.1590/S0066-782X2007000400022. PMID 17546284.
- 1 2 3 4 5 Lieb, Julian (2004). "The immunostimulating and antimicrobial properties of lithium and antidepressants". Journal of Infection. British Infection Association (Elsevier). 49 (2): 88–93. doi:10.1016/j.jinf.2004.03.006. ISSN 0163-4453. PMID 15236914.
- ↑ Krinsky, William L. (2002). Mullen, Gary; Durden, Lance (eds.). Medical and veterinary entomology. Amsterdam Boston: Academic Press. pp. 67–86/xv–597. doi:10.1016/B978-012510451-7/50007-4. ISBN 978-0-12-510451-7. OCLC 50752006. S2CID 82769743. ISBN 0125104510. ISBN 9780080536071.
- ↑ Telleria, Jenny; Tibayrenc, Michel, eds. (2017). American trypanosomiasis Chagas disease : one hundred years of research. Amsterdam, Netherlands: Elsevier. doi:10.1016/B978-0-12-801029-7.00007-1. ISBN 978-0-12-801029-7. OCLC 971022099. S2CID 82080107. ISBN 0128010290.
- ↑ Sant’Anna, Maurício Roberto Viana; Soares, Adriana Coelho; Araujo, Ricardo Nascimento; Gontijo, Nelder Figueiredo; Pereira, Marcos Horácio (2017). "Triatomines (Hemiptera, Reduviidae) blood intake: Physical constraints and biological adaptations". Journal of Insect Physiology. Elsevier. 97: 20–26. doi:10.1016/j.jinsphys.2016.08.004. ISSN 0022-1910. PMID 27521585.
- ↑ Kohl, Linda; Bastin, Philippe (2005). "The Flagellum of Trypanosomes". In Jeon, Kwang W. (ed.). A Survey of Cell Biology (PDF). International Review of Cytology. Vol. 244. pp. 227–85. doi:10.1016/S0074-7696(05)44006-1. ISBN 978-0-08-045779-6. PMID 16157182. Archived (PDF) from the original on 2022-10-17. Retrieved 2022-10-02.
- 1 2 Ley, Victoria; Andrews, Norma W.; Robbins, Edith S.; Nussenzweig, Victor (1988). "Amastigotes of Trypanosoma cruzi sustain an infective cycle in mammalian cells". Journal of Experimental Medicine. 168 (2): 649–59. doi:10.1084/jem.168.2.649. PMC 2189010. PMID 3045248.
- ↑ Messenger LA, Miles MA (2015). "Evidence and importance of genetic exchange among field populations of Trypanosoma cruzi". Acta Trop. 151: 150–5. doi:10.1016/j.actatropica.2015.05.007. PMC 4644990. PMID 26188331.
- ↑ Berry, Alexander S. F.; Salazar-Sánchez, Renzo; Castillo-Neyra, Ricardo; Borrini-Mayorí, Katty; Chipana-Ramos, Claudia; Vargas-Maquera, Melina; Ancca-Juarez, Jenny; Náquira-Velarde, César; Levy, Michael Z.; Brisson, Dustin; Bartholomeu, Daniella Castanheira (20 May 2019). "Sexual reproduction in a natural Trypanosoma cruzi population". PLOS Neglected Tropical Diseases. 13 (5): e0007392. doi:10.1371/journal.pntd.0007392. PMC 6544315. PMID 31107905.
- ↑ Jansen, Ana Maria; Xavier, Samanta Cristina das Chagas; Roque, André Luiz Rodrigues (6 September 2018). "Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil". Parasites & Vectors. 11 (1): 502. doi:10.1186/s13071-018-3067-2. ISSN 1756-3305. Archived from the original on 21 June 2022. Retrieved 21 November 2022.
- ↑ Hemmige, Vagish; Tanowitz, Herbert; Sethi, Aisha (2012). "Trypanosoma cruzi infection: a review with emphasis on cutaneous manifestations". International journal of dermatology. 51 (5): 501–508. doi:10.1111/j.1365-4632.2011.05380.x. ISSN 0011-9059. Archived from the original on 24 June 2020. Retrieved 22 November 2022.Most patients with acute infection are asymptomatic or have mild symptoms. After an incubation period of 1–2 weeks, a minority of patients will experience a non-specific febrile illness. A newly infected individual may develop fever, chills, nausea, vomiting, diarrhea, rash, meningeal irritation, conjunctivitis, lymphadenopathy, or hepatosplenomegaly.
- ↑ "Chagas Disease | NIH". clinicalinfo.hiv.gov. Archived from the original on 2022-08-07. Retrieved 2022-11-27.
- ↑ "Chagas disease: MedlinePlus Medical Encyclopedia". medlineplus.gov. Archived from the original on 1 October 2008. Retrieved 27 November 2022.
- ↑ Bern, Caryn (30 July 2015). "Chagas' Disease". doi:10.1056/nejmra1410150. Archived from the original on 15 July 2022. Retrieved 23 November 2022.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Guarner, Jeannette (1 May 2019). "Chagas disease as example of a reemerging parasite". Seminars in Diagnostic Pathology. 36 (3): 164–169. doi:10.1053/j.semdp.2019.04.008. ISSN 0740-2570. Retrieved 23 November 2022.
- ↑ Bain BJ (20 January 2015). Blood Cells: A Practical Guide. John Wiley & Sons. pp. 165–7. ISBN 978-1-118-81733-9. Archived from the original on 1 May 2021. Retrieved 6 August 2020.
- ↑ Bern, Caryn; Messenger, Louisa A.; Whitman, Jeffrey D.; Maguire, James H. (18 December 2019). "Chagas Disease in the United States: a Public Health Approach". Clinical Microbiology Reviews. 33 (1): e00023–19. doi:10.1128/CMR.00023-19. ISSN 0893-8512. Archived from the original on 22 September 2022. Retrieved 23 November 2022.
- ↑ Schijman AG (August 2018). "Molecular diagnosis of Trypanosoma cruzi". Acta Tropica. 184: 59–66. doi:10.1016/j.actatropica.2018.02.019. hdl:11336/79861. PMID 29476727.
- ↑ Quinde-Calderón, Leonardo; Rios-Quituizaca, Paulina; Solorzano, Luis; Dumonteil, Eric (2016). "Ten years (2004–2014) of Chagas disease surveillance and vector control in Ecuador: Successes and challenges" (PDF). Tropical Medicine & International Health. 21 (1): 84–92. doi:10.1111/tmi.12620. PMID 26458237. S2CID 25754153. Archived (PDF) from the original on 2021-12-16. Retrieved 2022-10-02.
- ↑ "CDC Works 24/7". Centers for Disease Control and Prevention. Archived from the original on 2006-03-31. Retrieved 2016-04-16.
- ↑ Stevens, Lori; Dorn, Patricia L.; Hobson, Julia; de la Rua, Nicholas M.; Lucero, David E.; Klotz, John H.; Schmidt, Justin O.; Klotz, Stephen A. (2012). "Vector Blood Meals and Chagas Disease Transmission Potential, United States". Emerging Infectious Diseases. 18 (4): 646–649. doi:10.3201/eid1804.111396. PMC 3309679. PMID 22469536.
- ↑ Xavier, Samanta Cristina das Chagas; Roque, André Luiz Rodrigues; Bilac, Daniele; de Araújo, Vitor Antônio Louzada; Neto, Sócrates Fraga da Costa; Lorosa, Elias Seixas; da Silva, Luiz Felipe Coutinho Ferreira; Jansen, Ana Maria (22 May 2014). "Distantiae Transmission of Trypanosoma cruzi: A New Epidemiological Feature of Acute Chagas Disease in Brazil". PLoS Neglected Tropical Diseases. 8 (5): e2878. doi:10.1371/journal.pntd.0002878. ISSN 1935-2727. Archived from the original on 25 March 2022. Retrieved 23 November 2022.
- ↑ Reisenman, Carolina E.; Lawrence, Gena; Guerenstein, Pablo G.; Gregory, Teresa; Dotson, Ellen; Hildebrand, John G. "Infection of Kissing Bugs with Trypanosoma cruzi, Tucson, Arizona, USA - Volume 16, Number 3—March 2010 - Emerging Infectious Diseases journal - CDC". doi:10.3201/eid1603.090648. Archived from the original on 6 October 2022. Retrieved 24 November 2022.
{{cite journal}}: Cite journal requires|journal=(help) - ↑ Monteiro, Fernando Araujo; Weirauch, Christiane; Felix, Márcio; Lazoski, Cristiano; Abad-Franch, Fernando (2018). "Evolution, Systematics, and Biogeography of the Triatominae, Vectors of Chagas Disease". Advances in Parasitology. Vol. 99. pp. 265–344. doi:10.1016/bs.apar.2017.12.002. ISBN 978-0-12-815192-1. PMID 29530308.
- ↑ Reis-Cunha, João Luís; Baptista, Rodrigo P.; Rodrigues-Luiz, Gabriela F.; Coqueiro-dos-Santos, Anderson; Valdivia, Hugo O.; de Almeida, Laila Viana; Cardoso, Mariana Santos; D’Ávila, Daniella Alchaar; Dias, Fernando Hugo Cunha; Fujiwara, Ricardo Toshio; Galvão, Lúcia M. C.; Chiari, Egler; Cerqueira, Gustavo Coutinho; Bartholomeu, Daniella C. (13 November 2018). "Whole genome sequencing of Trypanosoma cruzi field isolates reveals extensive genomic variability and complex aneuploidy patterns within TcII DTU". BMC Genomics. 19 (1): 816. doi:10.1186/s12864-018-5198-4. PMC 6234542. PMID 30424726.
- ↑ GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (November 2018). "Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017". Lancet. 392 (10159): 1789–1858. doi:10.1016/S0140-6736(18)32279-7. PMC 6227754. PMID 30496104.
- ↑ GBD 2017 Causes of Death Collaborators (November 2018). "Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017". Lancet. 392 (10159): 1736–88. doi:10.1016/S0140-6736(18)32203-7. PMC 6227606. PMID 30496103.
- ↑ "Chagas disease (American trypanosomiasis)". web.archive.org. 20 January 2020. Archived from the original on 20 January 2020. Retrieved 19 November 2022.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - 1 2 Moncayo A, Silveria AC (2017). "Current epidemiological trends of Chagas disease in Latin America and future challenges: epidemiology, surveillance, and health policies". American Trypanosomiasis – Chagas Disease (2 ed.). Elsevier. pp. 59–88. doi:10.1016/B978-0-12-801029-7.00004-6.
- ↑ "The Southern Cone Initiative: an update". Special Programme for Research and Training in Tropical Diseases (TDR) (Press release). WHO. 2004. Archived from the original on 22 September 2009. Retrieved 29 August 2008.
- ↑ Grillet ME, Hernández-Villena JV, Llewellyn MS, et al. (May 2019). "Venezuela's humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region" (PDF). Lancet Infect Dis (Review). 19 (5): e149–e161. doi:10.1016/S1473-3099(18)30757-6. PMID 30799251. Archived (PDF) from the original on 9 March 2020. Retrieved 6 August 2020.
- ↑ Alarcón de Noya B, Noya Gonzáles O (9 September 2019). "Orally Transmitted Chagas Disease: Biology, Epidemiology, and Clinical Aspects of a Foodborne Infection". In Marcelo Altcheh J, Freilij H (ed.). Chagas Disease: A Clinical Approach. Birkhäuser Advances in Infectious Diseases. Switzerland: Springer Nature. pp. 225–241. doi:10.1007/978-3-030-00054-7_11. ISBN 978-3-030-00054-7. ISSN 2504-3811.
- ↑ Hotez PJ, Basáñez MG, Acosta-Serrano A, Grillet ME (2017). "Venezuela and its rising vector-borne neglected diseases". PLOS Neglected Tropical Diseases. 11 (6): e0005423. doi:10.1371/journal.pntd.0005423. ISSN 1935-2735. PMC 5490936.
- ↑ Grillet ME, Hernández-Villena JV, Llewellyn MS, et al. (May 2019). "Venezuela's humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region" (PDF). Lancet Infect Dis (Review). 19 (5): e149–e161. doi:10.1016/S1473-3099(18)30757-6. PMID 30799251. Archived (PDF) from the original on 2020-03-09. Retrieved 2022-11-19.
- ↑ Morel CM, Lazdins J (October 2003). "Chagas disease". Nat Rev Microbiol. 1 (1): 14–5. doi:10.1038/nrmicro735. PMID 15040175.
- ↑ Jansen AM, Roque ALR (2010). "11 - Domestic and Wild Mammalian Reservoirs". In Telleria J, Tibayrenc M (eds.). American Trypanosomiasis. Elsevier. pp. 249–276. doi:10.1016/B978-0-12-384876-5.00011-3. ISBN 978-0-123-84876-5.
External links
- "American Trypanosomiasis (Trypanosoma cruzi)". DPDx—Laboratory Identification of Parasitic Diseases of Public Health Concern. Centers for Disease Control and Prevention. 29 November 2013. Archived from the original on 28 October 2022. Retrieved 2 October 2022.
- "Trypanosoma cruzi". NCBI Taxonomy Browser. 5693. Archived from the original on 2021-12-17. Retrieved 2022-10-02.