Cutaneous leishmaniasis
| Cutaneous leishmaniasis | |
|---|---|
| Other names: Oriental sore, Tropical sore, Chiclero ulcer, Chiclero's ulcer, Aleppo boil, Delhi Boil or Desert boil[1][2][3] | |
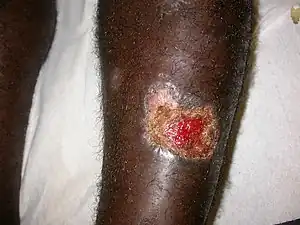 | |
| Cutaneous leishmaniasis in a man from French Guiana | |
| Specialty | Infectious disease |
| Symptoms | Swallowing difficulty,skin ulcer,nosebleeds |
| Causes | About thirty species of Leishmania |
| Diagnostic method | PCR test, clinical presentation |
| Treatment | It depends which species has caused the condition |
Cutaneous leishmaniasis ( or Chiclero's ulcer[1])is the most common form of leishmaniasis affecting humans.[4] It is a skin infection caused by a single-celled parasite that is transmitted by the bite of a phlebotomine sand fly. There are many species of Leishmania that may cause cutaneous leishmaniasis.[5]
This disease is considered to be a zoonosis (an infectious disease that is naturally transmissible from animals to humans), with the exception of Leishmania tropica — which is often an anthroponotic disease (an infectious disease that is naturally transmissible from humans to vertebrate animals).[3]The most common causes by Old World species are L. major, L. tropica, and L. aethiopica;while the New World species, has L. mexicana ans L. Viannia.[6]
Signs and symptoms
The presentation for Cutaneous leishmaniasis is as follows:[7]
- Swallowing difficulty
- Skin ulcer
- Nosebleeds
- Skin sores
- Runny nose
- Erosion in the mouth and inner nose
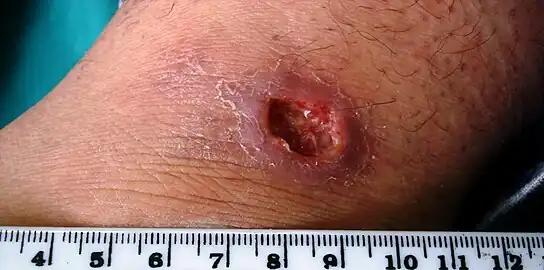 Cutaneous leishmaniasis
Cutaneous leishmaniasis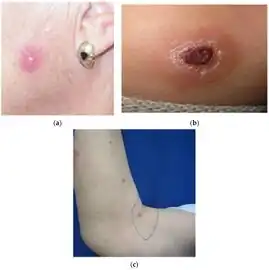 a-c)Types of Cutaneous leishmaniasis
a-c)Types of Cutaneous leishmaniasis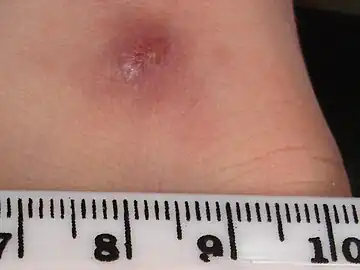 Cutaneous leishmaniasis
Cutaneous leishmaniasis
Cause
Leishmania is a genus of trypanosomes that are responsible for the disease leishmaniasis.[8][9][10] Leishmania species are unicellular eukaryotes having a well-defined nucleus and other cell organelles including kinetoplasts and flagella. Depending on the stage of their life cycle, they exist in two structural variants[11]
They are spread by sandflies of the genus Phlebotomus in the Old World, and of the genus Lutzomyia in the New World. At least 93 sandfly species are proven or probable vectors worldwide.[12]
Their primary hosts are vertebrates; Leishmania commonly infects dogs, sloths, rodents, and humans.[13]
Pathophysiology
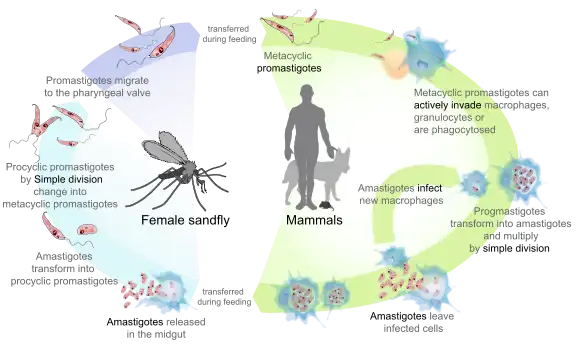
Promastigotes of Leishmania are transmitted to human skin by the bite of a sandfly. Leishmania then invades human macrophages and replicates intracellularly. A raised, red lesion develops at the site of the bite. The lesion then ulcerates and may become secondarily infected with bacteria. In many species (for example, L. major) the lesion often spontaneously heals with atrophic scarring. In some species (for example, L. braziliensis) the lesion may spontaneously heal with scarring, but then reappear elsewhere (especially as destructive mucocutaneous lesions). Lesions of other Leishmania species may spontaneously heal and then reappear as satellite lesions around the site of the original lesion, or along the route of lymphatic drainage.[14][15][16][17]
Some species tend to cause cutaneous leishmaniasis, whereas some species tend to cause visceral leishmaniasis, though emerging research, due to high deployment rates of western countries to indigenous areas, is showing these species specific presentation lines are blurring.[18][14][15]
Diagnosis
Diagnosis is based on the characteristic appearance of non-healing raised, scaling lesions that may ulcerate and become secondarily infected with organisms such as Staphylococcus aureus, in someone who has returned from an endemic area[19].[20][21]
In resource limited settings, fine-needle aspiration of the lesion is confirmatory with identification of amastigote form of Leishmania.[22] The gold standard for diagnosis is a PCR test.[23]
Prevention
The sand fly stings mainly at night, and it usually occurs about half a meter above the ground. To avoid the possibility of stinging, one should apply mosquito repellent, and cover the body.[24]
Studies conducted in recent years show that the plant Bougainvillea glabra may protect against the sand fly. The plant was found to be toxic to sand flies and that the life span of flies that ate from this plant was significantly shortened and sometimes led to their premature death before they could spread the disease.[25][26]Hebrew University study found that some plants attract sand flies. These plants often attract sand flies up to 14 times more than Bougainvillea glabra, but unlike Bougainvillea glabra, are not toxic to the sand flies. Based on this information, the dispersion of sand flies can be controlled by limiting the growth of these plants near populated areas. Alternatively, these plants may serve to capture and control sand flies by using their odor compounds or the plants themselves alongside simple glue traps, or by spraying them with deadly pesticides for sand flies which are safe for humans and mammals (e.g., boric acid or spinosad) thereby stopping the spread of the disease. Of the dozens of plants examined, the three plants that attracted especially sand flies are the Ochradenus baccatus, Prosopis farcta, and Tamarix nilotica.[27]
Treatment
New World cutaneous leishmaniasis
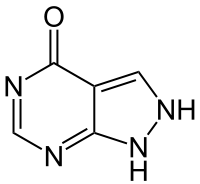
Pentavalent antimonial drugs (sodium stibogluconate (SSG) and meglumine antimonate have been used since the 1940s, but they are expensive, toxic, and painful.[28] Treatments that work for one species of Leishmania may not work for another; therefore, it is recommended that the exact species be identified prior to initiating treatment. Unfortunately, leishmaniasis is an orphan disease in developed nations, and almost all the current treatment options are toxic with significant side effects.[28][29][30]
The best-studied treatments for ACML caused by two Leishmania species are listed below. However, one should note that most of the studies examining treatments of ACML were poorly designed. Therefore, no definitive treatment guidelines or recommendations are currently available, as large-scale and well-conducted studies are necessary to evaluate the long-term effects of current treatments.[28]
- Leishmania braziliensis and Leishmania panamensis: There is good evidence that oral allopurinol plus intramuscular MA is superior to either medication alone. In addition, a 20-day course of intravenous MA was better than a 7-day course as well as a 3- or 7-day course of intravenous MA with paromomycin + 12% methylbenzethonium chloride.[28]
- For L. braziliensis, oral pentoxifylline plus intravenous SSG seems to be more efficacious than intravenous SSG alone, and intravenous MA was superior to intramuscular paromomycin sulfate and IV pentamidine. Likewise, intramuscular MA was shown to be better than the Bacillus Calmette-Guerin vaccine.[28]
- For L. panamensis, oral ketoconazole, oral miltefosine, and topical paromomycin + 12% methylbenzethonium chloride were shown to be superior to placebo.[28]
There is no strong evidence for the efficacy of surgery, oral itraconazole and fluconazole, oral antibiotics (rifampicin, metronidazole, cotrimoxazole), intravenous or topical amphotericin B, oral dapsone, photodynamic therapy, promoting healing therapies, laser, or cryotherapy treatments.[28]
Old World cutaneous leishmaniasis
Similar to ACML, the treatment recommendations for Old World cutaneous leishmaniasis (OWCL) are uncertain due to the variability of and inconsistencies within the research.[31]
Most studies done to assess treatments of OWCL included two species of parasites, Leishmania major and Leishmania tropica. The most well-studied treatments for OWCL are oral itraconazole and topical paromomycin.[31]
People treated with oral itraconazole for an average of 2.5 months had a higher cure rate compared to placebo, but they also had a higher rate of side effects, including gastrointestinal complaints, abnormal liver function, headaches, and dizziness.[31]
People treated with topical paromomycin showed no difference in cure rate compared to placebo, but patients treated with paromomycin had a higher rate of adverse skin reactions.[31]
The treatments for other Leishmania species responsible for OWCL, such as L. infantum, L. aethiopica, and L. donovani, have not been thoroughly studied. In addition, the effects of leishmaniasis treatment in children, women of childbearing age, patients with comorbidities, and immunocompromised patients have not been well established.[31]
Epidemiology
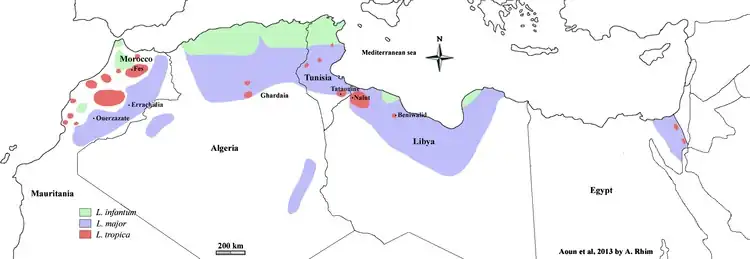
Cutaneous leishmaniasis is endemic in all tropical and subtropical areas of the world.The distribution of this disease is very tightly linked to geography, and villages even several miles apart can have very different rates of cutaneous leishmaniasis.[33][32]
Most species of Leishmania are capable of infecting humans and causing cutaneous leishmaniasis. In the New World, these organisms include L. amazonensis, L. braziliensis, L. guyanensis, L. lainsoni, L. lindenbergi,[34] L. mexicana, L. naiffi, L. panamensis, L. peruviana, L. shawi, and L. venezuelensis. Old World species that cause cutaneous leishmaniasis include L. aethiopica, L. infantum, L. major, and L. tropica. With the exception of L. tropica — which is commonly associated with human settlements and therefore considered to be an anthroponotic species — all of these organisms are zoonotic.[3] As demographic changes occur in developing nations, some species that have traditionally been considered to be zoonotic (e.g., L. panamensis) are becoming primarily human pathogens.[35]
Dogs and rodents serve as the primary animal reservoir hosts in the sylvatic cycle, but people with chronic PKDL can also serve as important reservoir hosts for cutaneous leishmaniasis.[36] The most common vectors for cutaneous leishmaniasis in the Old World are sandflies of the genus Phlebotomus, while Lutzomyia and those within the family Psychodidae are the most common vectors in the New World. There are more than 600 species of phlebotomine sandflies, and only 30 of these are known vectors.[37] Cutaneous leishmaniasis has been seen in American and Canadian troops coming back from Afghanistan.[38]
Outbreak in 2016
The Middle East, in 2016, seems to be experiencing an increase in the cutaneous leishmaniasis disease due to migrants fleeing the Islamic State of Iraq and the Levant. Reports of the increase in the disease have surfaced in Turkey, Lebanon, and elsewhere.[39][40]
The huge increase in the spread of the disease is attributed to the refugee crises in the Middle East and North Africa over the past five years, particularly due to the displacement of millions of Syrian refugees.[41] The outbreak among Syrian refugees was documented by the World Health Organization (WHO) in 2012 and recognized as ongoing.[42]
A recent study with large series of cases from Mid-western region of Nepal have demonstrated that cutaneous leishmaniasis is an under recognized medical condition posing health challenges mandating new guidelines for its elimination/ eradication.[22]
Research
A 2015 review article claimed that the development and use of a vaccine would be the best way to eliminate leishmaniasis from South Asia.[43] Attempts to create a vaccine with live, inactivated or attenuated Leishmania have failed.[44] Attempts to create a peptide, DNA, or protein vaccine have shown efficacy in animal vaccine models but not effective in humans.[44] There are a series of challenges with explanations in molecular biology which explain the difficulty of vaccine development.[44]
Vaccine development is difficult because the parasites live in humans, sandflies, and other animals, so a vaccine in humans alone would not eliminate the protozoan in insects and animals.[45] There is a challenge in interpreting the data in animal models to apply to humans.[46] Another challenge is effectively transferring knowledge from laboratory settings to field practice.[46] There is also a basic lack of scientific understanding of how an antiparasitic vaccine should generate and maintain immunological memory during parasitic infection.[46]The development of a vaccine using CRISPR-Cas9 technology was published in 2020[47] which showed that inoculation with a live attenuated Leishmania major strain induces durable protection, analogous to leishmanization. Another gene deletion mutant was created in a Leishmania mexicana strain in 2022, showing complete inhibition of the typical cutaneous lesions in mouse models thanks to a diminished induction of the Th2 cytokines.[48]
Other animals

Besides humans, cutaneous leishmaniasis often affects other animals, notably in dogs as canine leishmaniasis.[3]
Canine leishmaniasis was first identified in Europe in 1903, and in 1940, 40% of all dogs in Rome were determined to be positive for leishmaniasis.[49]
Traditionally thought of as a disease only found near the Mediterranean basin, 2008 research claims new findings are evidence that canine leishmaniasis is currently expanding in continental climate areas of northwestern Italy, far from the recognized disease-endemic areas along the Mediterranean coasts.[50]
Cases of leishmaniasis began appearing in North America in 2000,[51] and, as of 2008, Leishmania-positive foxhounds have been reported in 22 U.S. states and two Canadian provinces.[52]
References
- 1 2 Calvopiña M, Martinez L, Hashiguchi Y (August 2013). "Cutaneous leishmaniasis "chiclero's ulcer" in subtropical Ecuador". The American Journal of Tropical Medicine and Hygiene. 89 (2): 195–6. doi:10.4269/ajtmh.12-0690. PMC 3741233. PMID 23926136.
- ↑ Stowers JH (1920). "Case of Delhi Boil or Sore (Syn.: Oriental Sore; Aleppo Boil)". Proceedings of the Royal Society of Medicine. 13 (Dermatol Sect): 81–3. doi:10.1177/003591572001300351. PMC 2152205. PMID 19980989.
- 1 2 3 4 The Institute for International Cooperation in Animal Biologics and the Center for Food Security and Public Health (October 2009). "Leishmaniasis (cutaneous and visceral)" (PDF). Ames, Iowa: College of Veterinary Medicine, Iowa State University. Archived (PDF) from the original on 2014-11-25. Retrieved 2015-01-04.
- ↑ James WD, Berger TG (2006). Andrews' Diseases of the Skin: clinical Dermatology. Saunders Elsevier. p. 423. ISBN 978-0-7216-2921-6.
- ↑ "Cutaneous and Mucosal Leishmaniasis - PAHO/WHO | Pan American Health Organization". www.paho.org. Archived from the original on 27 March 2023. Retrieved 4 July 2023.
- ↑ Tripathi, G. (March 2010). Cellular and Biochemical Science. I. K. International Pvt Ltd. p. 1084. ISBN 978-81-88237-85-2. Archived from the original on 9 July 2023. Retrieved 7 July 2023.
- ↑ "Leishmaniasis: MedlinePlus Medical Encyclopedia". medlineplus.gov. Archived from the original on 24 March 2023. Retrieved 25 June 2023.
- ↑ Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 749–54. ISBN 0-8385-8529-9.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Myler P; Fasel N (editors) (2008). Leishmania: After The Genome. Caister Academic Press. ISBN 978-1-904455-28-8.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ↑ Ansari MY, Equbal A, Dikhit MR, Mansuri R, Rana S, Ali V, Sahoo GC, Das P (Nov 2015). "Establishment of Correlation between In-Silico &In-Vitro Test Analysis against Leishmania HGPRT to inhibitors". International Journal of Biological Macromolecules. 83: 78–96. doi:10.1016/j.ijbiomac.2015.11.051. PMID 26616453.
- ↑ Pulvertaft, RJ; Hoyle, GF (1960). "Stages in the life-cycle of Leishmania donovani". Transactions of the Royal Society of Tropical Medicine and Hygiene. 54 (2): 191–6. doi:10.1016/0035-9203(60)90057-2. PMID 14435316.
- ↑ WHO (2010) Annual report. Geneva
- ↑ Handman, Emanuela (April 2001). "Leishmaniasis: Current Status of Vaccine Development". Clinical Microbiology Reviews. 14 (2): 229–243. doi:10.1128/CMR.14.2.229-243.2001. ISSN 0893-8512. Archived from the original on 3 July 2023. Retrieved 2 July 2023.
- 1 2 Volpedo, Greta; Pacheco-Fernandez, Thalia; Holcomb, Erin A.; Cipriano, Natalie; Cox, Blake; Satoskar, Abhay R. (2021). "Mechanisms of Immunopathogenesis in Cutaneous Leishmaniasis And Post Kala-azar Dermal Leishmaniasis (PKDL)". Frontiers in Cellular and Infection Microbiology. 11. doi:10.3389/fcimb.2021.685296/full. ISSN 2235-2988. Archived from the original on 2022-08-06. Retrieved 2023-06-27.
- 1 2 Reithinger, Richard; Dujardin, Jean-Claude; Louzir, Hechmi; Pirmez, Claude; Alexander, Bruce; Brooker, Simon (1 September 2007). "Cutaneous leishmaniasis". The Lancet Infectious Diseases. 7 (9): 581–596. doi:10.1016/S1473-3099(07)70209-8. ISSN 1473-3099. Archived from the original on 7 June 2023. Retrieved 30 June 2023.
- ↑ Torres-Guerrero, E; Quintanilla-Cedillo, MR; Ruiz-Esmenjaud, J; Arenas, R (2017). "Leishmaniasis: a review". F1000Research. 6: 750. doi:10.12688/f1000research.11120.1. PMID 28649370. Archived from the original on 10 April 2022. Retrieved 27 June 2023.
- ↑ Bates, Paul A. (May 2018). "Revising Leishmania's life cycle". Nature Microbiology. 3 (5): 529–530. doi:10.1038/s41564-018-0154-2. ISSN 2058-5276. Archived from the original on 21 May 2022. Retrieved 28 June 2023.
- ↑ Pottumarthy, SUDHA; Fritsche, THOMAS R. (1 January 2005). "Chapter 96 - Parasitic Infections of the Peripheral Nervous System". Peripheral Neuropathy (Fourth Edition). W.B. Saunders. pp. 2153–2176. ISBN 978-0-7216-9491-7. Retrieved 28 June 2023.
- ↑ Aronson, Naomi E.; Joya, Christie A. (March 2019). "Cutaneous Leishmaniasis: Updates in Diagnosis and Management". Infectious Disease Clinics of North America. 33 (1): 101–117. doi:10.1016/j.idc.2018.10.004. ISSN 1557-9824. Archived from the original on 12 October 2022. Retrieved 29 June 2023.
- ↑ de Vries, Henry J. C.; Schallig, Henk D. (November 2022). "Cutaneous Leishmaniasis: A 2022 Updated Narrative Review into Diagnosis and Management Developments". American Journal of Clinical Dermatology. 23 (6): 823–840. doi:10.1007/s40257-022-00726-8. Archived from the original on 3 July 2023. Retrieved 1 July 2023.
- ↑ Ziaie, H; Sadeghian, G (2008). "Isolation of bacteria causing secondary bacterial infection in the lesions of Cutaneous Leishmaniasis". Indian journal of dermatology. 53 (3): 129–31. doi:10.4103/0019-5154.43217. PMID 19882011. Archived from the original on 6 July 2023. Retrieved 3 July 2023.
- 1 2 Ghimire, Pragya Gautam; Shrestha, Richa; Pandey, Sumit; Pokhrel, Kumar; Pande, Rajan (2018-03-29). "Cutaneous Leishmaniasis: A Neglected Vector Borne Tropical Disease in Midwestern Region of Nepal". Nepal Journal of Dermatology, Venereology & Leprology. 16 (1): 41–44. doi:10.3126/njdvl.v16i1.19405. ISSN 2091-167X.
- ↑ Reithinger R, Dujardin JC (January 2007). "Molecular diagnosis of leishmaniasis: current status and future applications". Journal of Clinical Microbiology. 45 (1): 21–5. doi:10.1128/JCM.02029-06. PMC 1828971. PMID 17093038.
- ↑ Prevention, CDC-Centers for Disease Control and (19 February 2020). "CDC - Leishmaniasis - Prevention & Control". www.cdc.gov. Archived from the original on 30 March 2019. Retrieved 30 June 2023.
- ↑ Schlein Y, Jacobson RL, Müller GC (October 2001). "Sand fly feeding on noxious plants: a potential method for the control of leishmaniasis". The American Journal of Tropical Medicine and Hygiene. 65 (4): 300–3. doi:10.4269/ajtmh.2001.65.300. PMID 11693873.
- ↑ Kaldas RM, El Shafey AS, Shehata MG, Samy AM, Villinski JT (April 2014). "Experimental effect of feeding on Ricinus communis and Bougainvillea glabra on the development of the sand fly Phlebotomus papatasi (Diptera: Psychodidae) from Egypt". Journal of the Egyptian Society of Parasitology. 44 (1): 1–12. doi:10.12816/0006441. PMID 24961006. S2CID 44557398.
- ↑ Müller GC, Revay EE, Schlein Y (March 2011). "Relative attraction of the sand fly Phlebotomus papatasi to local flowering plants in the Dead Sea region". Journal of Vector Ecology. 36 Suppl 1: S187–94. doi:10.1111/j.1948-7134.2011.00130.x. PMID 21366774.
- 1 2 3 4 5 6 7 Pinart, Mariona; Rueda, José-Ramón; Romero, Gustavo As; Pinzón-Flórez, Carlos Eduardo; Osorio-Arango, Karime; Silveira Maia-Elkhoury, Ana Nilce; Reveiz, Ludovic; Elias, Vanessa M.; Tweed, John A. (27 August 2020). "Interventions for American cutaneous and mucocutaneous leishmaniasis". The Cochrane Database of Systematic Reviews. 2020 (8): CD004834. doi:10.1002/14651858.CD004834.pub3. ISSN 1469-493X. PMC 8094931. PMID 32853410.
- ↑ Tuon, F. F.; Amato, V. S.; Graf, M. E.; Siqueira, A. M.; Nicodemo, A. C.; Neto, V. Amato (2008). "Treatment of New World cutaneous leishmaniasis: a systematic review with a meta-analysis". Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. Centre for Reviews and Dissemination (UK). Archived from the original on 6 July 2023. Retrieved 3 July 2023.
- ↑ Mitropoulos, Panagiotis; Konidas, Pete; Durkin-Konidas, Mindy (1 August 2010). "New World cutaneous leishmaniasis: Updated review of current and future diagnosis and treatment". Journal of the American Academy of Dermatology. 63 (2): 309–322. doi:10.1016/j.jaad.2009.06.088. ISSN 0190-9622.
- 1 2 3 4 5 Heras-Mosteiro J, Monge-Maillo B, Pinart M, Lopez Pereira P, Reveiz L, Garcia-Carrasco E, Campuzano Cuadrado P, Royuela A, Mendez Roman I, López-Vélez R (December 2017). "Interventions for Old World cutaneous leishmaniasis". The Cochrane Database of Systematic Reviews. 2017 (12): CD005067. doi:10.1002/14651858.CD005067.pub5. PMC 6485999. PMID 29192424.
- 1 2 Aoun K, Bouratbine A (2014). "Cutaneous leishmaniasis in North Africa: a review". Parasite. 21: 14. doi:10.1051/parasite/2014014. PMC 3952656. PMID 24626301.
- ↑ Prevention, CDC-Centers for Disease Control and (18 February 2020). "CDC - Leishmaniasis - Epidemiology & Risk Factors". www.cdc.gov. Archived from the original on 15 June 2019. Retrieved 1 July 2023.
- ↑ Cantanhêde, Lilian Motta; Mattos, Cristiane Batista; de Souza Ronconi, Camila; Filgueira, Camila Patrício Braga; da Silva Júnior, Cipriano Ferreira; Limeira, Claudino; de Jesus Silva, Helen Paula; Ferreira, Gabriel Eduardo Melim; Porrozzi, Renato; Ferreira, Ricardo de Godoi Mattos; Cupolillo, Elisa (2019). "First report of Leishmania (Viannia) lindenbergi causing tegumentary leishmaniasis in the Brazilian western Amazon region". Parasite. 26: 30. doi:10.1051/parasite/2019030. ISSN 1776-1042. PMC 6532396. PMID 31120019.

- ↑ Vergel C, Palacios R, Cadena H, Posso CJ, Valderrama L, Perez M, Walker J, Travi BL, Saravia NG (August 2006). "Evidence for leishmania (viannia) parasites in the skin and blood of patients before and after treatment". The Journal of Infectious Diseases. 194 (4): 503–11. doi:10.1086/505583. PMID 16845635.
- ↑ Centers for Disease Control and Prevention (July 10, 2014). "Parasites - leishmaniasis". Resources for health professionals. Atlanta, Georgia: United States Department of Health and Human Services. Archived from the original on 2015-03-07. Retrieved 2015-01-04.
- ↑ Connolly MA, World Health Organization (2005). Communicable disease control in emergencies: a field manual. World Health Organization. pp. 152–. ISBN 978-92-4-154616-4. Retrieved 12 June 2016.
- ↑ "Canadian soldiers bring back Old World disease". Medical Post.
- ↑ Sims A (30 May 2016). "A disfiguring tropical disease is sweeping across the Middle East". The Independent. Archived from the original on 13 June 2016. Retrieved 12 June 2016.
- ↑ Hiddleston S (2016). "An old disease rears its ugly head". Nature Middle East. doi:10.1038/nmiddleeast.2016.82.
- ↑ Du R, Hotez PJ, Al-Salem WS, Acosta-Serrano A (May 2016). "Old World Cutaneous Leishmaniasis and Refugee Crises in the Middle East and North Africa". PLOS Neglected Tropical Diseases. 10 (5): e0004545. doi:10.1371/journal.pntd.0004545. PMC 4882064. PMID 27227772.
- ↑ Saroufim M, Charafeddine K, Issa G, Khalifeh H, Habib RH, Berry A, Ghosn N, Rady A, Khalifeh I (October 2014). "Ongoing epidemic of cutaneous leishmaniasis among Syrian refugees, Lebanon". Emerging Infectious Diseases. 20 (10): 1712–5. doi:10.3201/eid2010.140288. PMC 4193275. PMID 25279543.
- ↑ Engwerda, Christian R.; Matlashewski, G (July 2015). "Development of Leishmania vaccines in the era of visceral leishmaniasis elimination" (PDF). Transactions of the Royal Society of Tropical Medicine and Hygiene. 109 (7): 423–4. doi:10.1093/trstmh/trv039. PMID 26048873. Archived (PDF) from the original on 2020-03-22. Retrieved 2021-06-27.
- 1 2 3 Zutshi, Shubhranshu; Kumar, Sunil; Chauhan, Prashant; Bansode, Yashwant; Nair, Arathi; Roy, Somenath; Sarkar, Arup; Saha, Bhaskar (18 October 2019). "Anti-Leishmanial Vaccines: Assumptions, Approaches, and Annulments" (PDF). Vaccines. 7 (4): 156. doi:10.3390/vaccines7040156. PMC 6963565. PMID 31635276. Archived (PDF) from the original on 2020-02-09. Retrieved 2021-06-27.
- ↑ Garg, Ravendra; Dube, Anuradha (March 2006). "Animal models for vaccine studies for visceral leishmaniasis" (PDF). Indian Journal of Medical Research. 123 (3): 439–54. PMID 16778322. Archived (PDF) from the original on 2021-06-28. Retrieved 2021-06-27.
- 1 2 3 Kedzierski, Lukasz (May 2010). "Leishmaniasis Vaccine: Where are We Today?". Journal of Global Infectious Diseases. 2 (2): 177–85. doi:10.4103/0974-777X.62881. PMC 2889658. PMID 20606974.
- ↑ Zhang, Wen-Wei; Karmakar, Subir; Gannavaram, Sreenivas; Dey, Ranadhir; Lypaczewski, Patrick; Ismail, Nevien; Siddiqui, Abid; Simonyan, Vahan; Oliveira, Fabiano; Coutinho-Abreu, Iliano V.; DeSouza-Vieira, Thiago; Meneses, Claudio; Oristian, James; Serafim, Tiago D.; Musa, Abu; Nakamura, Risa; Saljoughian, Noushin; Volpedo, Greta; Satoskar, Monika; Satoskar, Sanika; Dagur, Pradeep K.; McCoy, J. Philip; Kamhawi, Shaden; Valenzuela, Jesus G.; Hamano, Shinjiro; Satoskar, Abhay R.; Matlashewski, Greg; Nakhasi, Hira L. (10 July 2020). "A second generation leishmanization vaccine with a markerless attenuated Leishmania major strain using CRISPR gene editing". Nature Communications. 11 (1): 3461. Bibcode:2020NatCo..11.3461Z. doi:10.1038/s41467-020-17154-z. PMID 32651371. S2CID 218571920.
- ↑ Volpedo, Greta; Pacheco-Fernandez, Thalia; Holcomb, Erin A.; Zhang, Wen-Wei; Lypaczewski, Patrick; Cox, Blake; Fultz, Rebecca; Mishan, Chelsea; Verma, Chaitenya; Huston, Ryan H.; Wharton, Abigail R.; Dey, Ranadhir; Karmakar, Subir; Oghumu, Steve; Hamano, Shinjiro; Gannavaram, Sreenivas; Nakhasi, Hira L.; Matlashewski, Greg; Satoskar, Abhay R. (2 March 2022). "Centrin-deficient Leishmania mexicana confers protection against New World cutaneous leishmaniasis". npj Vaccines. 7 (1): 32. doi:10.1038/s41541-022-00449-1. PMC 8891280. PMID 35236861.
- ↑ Dereure J., Pratlong F., Dedet, J.P (1999) Geographical distribution and the identification of parasites causing canine leishmaniasis in the Mediterranean Basin. Canine leishmaniasis: an update. Proceedings of the International Canine Leishmaniasis Forum. Barcelona, Spain
- ↑ Ferroglio E, Maroli M, Gastaldo S, Mignone W, Rossi L (October 2005). "Canine leishmaniasis, Italy". Emerging Infect. Dis. 11 (10): 1618–20. doi:10.3201/eid1110.040966. PMC 3366729. PMID 16318709. Archived from the original on 2009-11-05. Retrieved 2023-06-26.
- ↑ Monti, Dean (June 2000). "Hunters hounded as leishmaniasis is diagnosed in Foxhounds". J Am Vet Med Assoc. 216 (12): 1887, 1890. PMID 10863579. Archived from the original on 2008-09-06. Retrieved 2023-06-26.
- ↑ Rosypal, Alexa. (2005) Characterization of Canine Leishmaniasis in the United States: Pathogenesis, Immunological Responses, and Transmission of an American Isolate of Leishmania infantum Archived 2023-06-30 at the Wayback Machine. Veterinary Clinics of North America Small Animal Practice Journal. Blacksburg, VA.
External links
| Classification |
|---|