Vosoritide
 | |
| Names | |
|---|---|
| Trade names | Voxzogo |
| Other names | BMN-111 |
| Clinical data | |
| Main uses | Achondroplasia (dwarfism)[1] |
| Side effects | Injection site pain, vomiting, low blood pressure[1][2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Subcutaneous injection |
| Typical dose | 15 ug/kg OD[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C176H290N56O51S3 |
| Molar mass | 4102.78 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Vosoritide, sold under the brand name Voxzogo, is a medication used to treat achondroplasia (dwarfism).[1][2] Specifically it is used in those who are at least two to five years old and still growing.[2][1] It is given by injection under the skin.[2]
Common side effects include injection site swelling, redness, itching, or pain; vomiting; and low blood pressure.[1][2] Safety in pregnancy is unclear.[1] It works by binding to a specific receptor called natriuretic peptide receptor-B, which reduces FGFR3, and therefore increases bone growth.[2]
Vosoritide was approved for medical use in Europe and the United States in 2021.[2][1] In France it costs about 260,000€ per year as of 2022.[5] This amount in the United States costs about 330,000 USD.[6]
Medical uses
In the European Union, it is used for achondroplasia in people two years of age and older whose epiphyses are not closed.[2]
In the United States, it is used to increase growth in children five years of age and older with achondroplasia and open epiphyses (growth plates).[1][4]
Over a year it increases growth by 1.6 cm.[2]
Dosage
The usual dose is 15 microgram / kg once per day.[2]
Mechanism of action
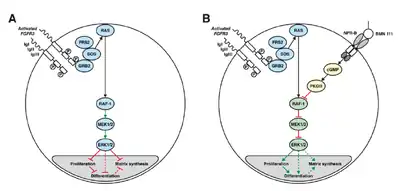
B: Vosoritide (BMN 111) blocks this mechanism by binding to the atrial natriuretic peptide receptor B (NPR-B), which subsequently inhibits the MAPK/ERK pathway at the RAF-1 protein.[7]
Achondroplasia is a genetic condition that causes severely short stature and disproportionate growth.[4] The average height of an adult with achondroplasia is approximately four feet.[4] People with achondroplasia have a genetic mutation that causes a certain growth regulation gene called fibroblast growth factor receptor 3 to be overly active, which prevents normal bone growth.[4]
Vosoritide works by binding to a receptor (target) called natriuretic peptide receptor type B (NPR-B), which reduces the activity of fibroblast growth factor receptor 3 (FGFR3).[2] FGFR3 is a receptor that normally down-regulates cartilage and bone growth when activated by one of the proteins known as acidic and basic fibroblast growth factor. It does so by inhibiting the development (cell proliferation and differentiation) of chondrocytes, the cells that produce and maintain the cartilaginous matrix which is also necessary for bone growth. Children with achondroplasia have one of several possible FGFR3 mutations resulting in constitutive (permanent) activity of this receptor, resulting in overall reduced chondrocyte activity and thus bone growth.[7]
The protein C-type natriuretic peptide (CNP), naturally found in humans, reduces the effects of over-active FGFR3. Vosoritide is a CNP analogue with the same effect but prolonged half-life,[7] allowing for once-daily administration.[8]
Chemistry
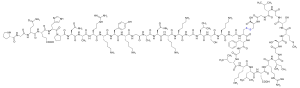
Vosoritide is an analog of CNP. It is a peptide consisting of the amino acids proline and glycine plus the 37 C-terminal amino acids from natural human CNP. The complete peptide sequence is
with a disulfide bridge between positions 23 and 39 (underlined).[9] The drug must be administered by injection as it would be rendered ineffective by the digestive system if taken by mouth.
History
Vosoritide was developed by BioMarin Pharmaceutical and, being the only available causal treatment for this condition, got orphan drug status in the US as well as the European Union.[2][10][11] It was in Phase II clinical trials in around 2015.[12][8]
The safety and efficacy of vosoritide in improving growth were evaluated in a year-long, double-blind, placebo-controlled, phase III study in participants five years and older with achondroplasia who have open epiphyses.[4] In the study, 121 participants were randomly assigned to receive either vosoritide injections under the skin or a placebo.[4] Researchers measured the participants' annualized growth velocity, or rate of height growth, at the end of the year.[4] Participants who received vosoritide grew an average 1.57 centimeters taller compared to those who received a placebo.[4] The U.S. Food and Drug Administration (FDA) granted the approval of Voxzogo to BioMarin.
Society and culture
Controversy
Some people with achondroplasia, as well as parents of children with this condition, have reacted to vosoritide's study results by saying that dwarfism is not a disease and consequently does not need treatment.[13]
Research
Vosoritide has resulted in increased growth in a clinical trial with 26 children. The ten children receiving the highest dose grew 6.1 centimetres (2.4 in) in six months, compared to 4.0 centimetres (1.6 in) in the six months before the treatment (p=0.01).[14] The body proportions, more specifically the ratio of leg length to upper body length – which is lower in achondroplasia patients than in the average population – was not improved by vosoritide, but not worsened either.[12][15]
As of September 2015, it is not known whether the effect of the drug will last long enough to result in normal body heights,[15] or whether it will reduce the occurrence of achondroplasia associated problems such as ear infections, sleep apnea or hydrocephalus. This, together with the safety of higher doses, is to be determined in further studies.[8]
References
- 1 2 3 4 5 6 7 8 9 "Voxzogo 0.4MG- vosoritide kit Voxzogo 0.56MG- vosoritide kit Voxzogo 1.2MG- vosoritide kit". DailyMed. Archived from the original on 19 December 2021. Retrieved 19 December 2021.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 "Voxzogo EPAR". European Medicines Agency. 23 June 2021. Archived from the original on 10 September 2021. Retrieved 9 September 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- 1 2 "Voxzogo APMDS". Therapeutic Goods Administration (TGA). 4 August 2022. Archived from the original on 6 August 2022. Retrieved 6 August 2022.
- 1 2 3 4 5 6 7 8 9 "FDA Approves First Drug to Improve Growth in Children with Most Common Form of Dwarfism". U.S. Food and Drug Administration (FDA) (Press release). 19 November 2021. Archived from the original on 19 November 2021. Retrieved 19 November 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Vosoritide". SPS - Specialist Pharmacy Service. 19 December 2019. Archived from the original on 24 October 2022. Retrieved 24 October 2022.
- ↑ "Voxzogo". Goodrx. Archived from the original on 24 October 2022. Retrieved 24 October 2022.
- 1 2 3 Lorget F, Kaci N, Peng J, Benoist-Lasselin C, Mugniery E, Oppeneer T, et al. (December 2012). "Evaluation of the therapeutic potential of a CNP analog in a Fgfr3 mouse model recapitulating achondroplasia". American Journal of Human Genetics. 91 (6): 1108–14. doi:10.1016/j.ajhg.2012.10.014. PMC 3516592. PMID 23200862.
- 1 2 3 Clinical trial number NCT02055157 for "A Phase 2 Study of BMN 111 to Evaluate Safety, Tolerability, and Efficacy in Children With Achondroplasia (ACH)" at ClinicalTrials.gov
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN): List 112" (PDF). WHO Drug Information. 28 (4): 539. 2014.
- ↑ "European Commission Approves BioMarin's Voxzogo (vosoritide) for the Treatment of Children with Achondroplasia from Age 2 Until Growth Plates Close" (Press release). BioMarin Pharmaceutical. 27 August 2021. Archived from the original on 28 August 2021. Retrieved 9 September 2021 – via PR Newswire.
- ↑ "Food and Drug Administration Accepts BioMarin's New Drug Application for Vosoritide to Treat Children with Achondroplasia" (Press release). BioMarin Pharmaceutical. 2 November 2020. Archived from the original on 10 September 2021. Retrieved 9 September 2021 – via PR Newswire.
- 1 2 Spreitzer H (6 July 2015). "Neue Wirkstoffe – Vosoritid". Österreichische Apothekerzeitung (in German) (14/2015): 28.
{{cite journal}}: CS1 maint: unrecognized language (link) - ↑ Pollack A (17 June 2015). "Drug Accelerated Growth in Children With Dwarfism, Pharmaceutical Firm Says". The New York Times. Archived from the original on 1 October 2015. Retrieved 3 March 2017.
- ↑ "BMN 111 (vosoritide) Improves Growth Velocity in Children With Achondroplasia in Phase 2 Study". BioMarin. 17 June 2015. Archived from the original on 9 September 2015. Retrieved 12 September 2015.
- 1 2 "Vosoritid" (in Deutsch). Arznei-News.de. 20 June 2015. Archived from the original on 6 March 2016. Retrieved 10 September 2015.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- Clinical trial number NCT03197766 for "A Study to Evaluate the Efficacy and Safety of BMN 111 in Children With Achondroplasia" at ClinicalTrials.gov