This article was co-authored by Chris Hasegawa, PhD and by wikiHow staff writer, Jennifer Mueller, JD. Dr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
There are 8 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 109,349 times.
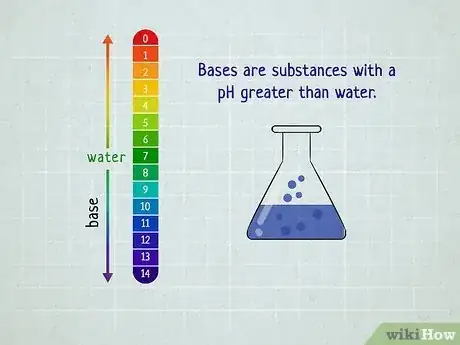
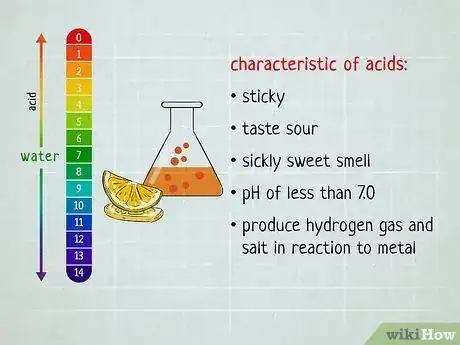
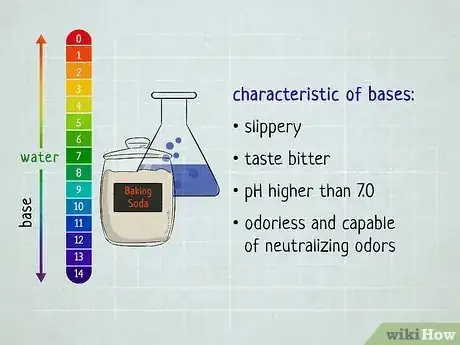
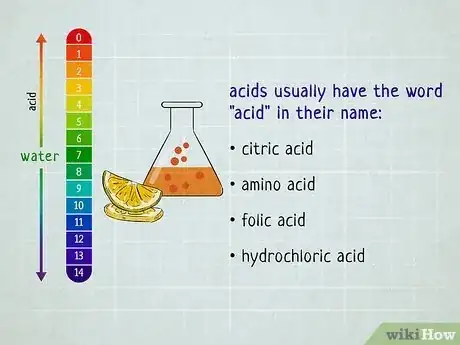
In chemistry, the easiest way to distinguish acids and bases is to look at the substance's pH level—acids have a pH of less than 7 while bases have a pH greater than 7.[1] But what if you don't happen to have any litmus paper handy? Turns out there are plenty of ways to distinguish between acids and bases that don't require testing the substance. You just have to evaluate its basic properties. Read on to find out how you can easily distinguish between acids and bases—no lab equipment necessary.
Steps
Expert Q&A
-
QuestionWhat is the major difference between acid and base?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean Ultimately, you'll notice notice a difference in the number of ions between acidic and basic solutions.
Ultimately, you'll notice notice a difference in the number of ions between acidic and basic solutions. -
QuestionHow do you introduce the topic of acids and bases?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean Approach the topic hands-on! For instance, you could teach your students how to dilute both acidic and basic solutions.
Approach the topic hands-on! For instance, you could teach your students how to dilute both acidic and basic solutions. -
QuestionWhat color will methyl orange be when it comes into contact with an acid or base?
 HanCommunity AnswerMethyl orange will turn red below a pH of 3.1, orange between 3.1 and 4.4, and yellow above 4.4.
HanCommunity AnswerMethyl orange will turn red below a pH of 3.1, orange between 3.1 and 4.4, and yellow above 4.4.
Warnings
- Strong acids and strong bases are both harmful and corrosive. Always protect your skin and eyes when experimenting with acids and bases.[14]⧼thumbs_response⧽
References
- ↑ https://flexbooks.ck12.org/cbook/ck-12-biology-flexbook-2.0/section/1.20/primary/lesson/acids-and-bases-in-biology-bio/
- ↑ https://courses.lumenlearning.com/boundless-chemistry/chapter/acids-and-bases/
- ↑ https://courses.lumenlearning.com/boundless-chemistry/chapter/acids-and-bases/
- ↑ https://courses.lumenlearning.com/cheminter/chapter/properties-of-acids-and-bases/
- ↑ https://www.pleasantvalleysd.org/cms/lib/CA00000039/Centricity/Domain/5846/6.3%20acids%20and%20bases.pdf
- ↑ https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Principles_of_Modern_Chemistry_(Oxtoby_et_al.)/Unit_4%3A_Equilibrium_in_Chemical_Reactions/15%3A_AcidBase_Equilibria/15.1%3A_Classifications_of_Acids_and_Bases
- ↑ https://www.pleasantvalleysd.org/cms/lib/CA00000039/Centricity/Domain/5846/6.3%20acids%20and%20bases.pdf
- ↑ https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch11/acidbase.php
- ↑ https://flexbooks.ck12.org/cbook/cbse-chemistry-class-10/section/2.4/primary/lesson/chemical-properties-of-acids-and-bases/
- ↑ https://www.pleasantvalleysd.org/cms/lib/CA00000039/Centricity/Domain/5846/6.3%20acids%20and%20bases.pdf
- ↑ https://www.mcgill.ca/oss/article/general-science-you-asked/can-baking-soda-really-absorb-odors-fridge
- ↑ https://www.pleasantvalleysd.org/cms/lib/CA00000039/Centricity/Domain/5846/6.3%20acids%20and%20bases.pdf
- ↑ https://www.pleasantvalleysd.org/cms/lib/CA00000039/Centricity/Domain/5846/6.3%20acids%20and%20bases.pdf
- ↑ https://flexbooks.ck12.org/cbook/ck-12-biology-flexbook-2.0/section/1.20/primary/lesson/acids-and-bases-in-biology-bio/