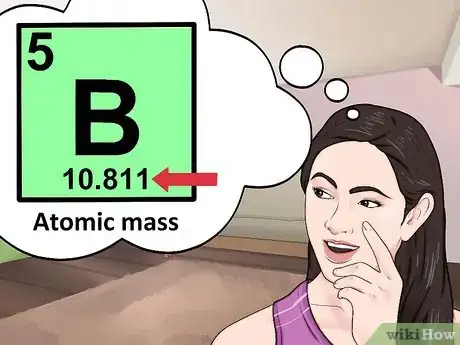This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
There are 8 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. This article received 116 testimonials and 81% of readers who voted found it helpful, earning it our reader-approved status.
This article has been viewed 2,816,989 times.
Finding the number of protons, neutrons, and electrons in a given element isn't as hard as it sounds. Oftentimes part of your answer will be right in front of you in the periodic table! Once you know where to look, finding the number of protons, neutrons, and electrons will be a breeze.
Steps
Calculating Protons, Electrons, and Neutrons
-
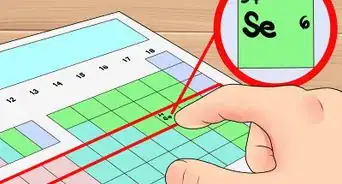
1Get a periodic table of elements. The periodic table is a chart that organizes elements by their atomic structure. It is color-coded and assigns each element a unique 1 or 2-letter abbreviation. Other elemental information includes atomic weight and atomic number.[1]
- You can find a periodic table online or in a chemistry book.
- In tests, normally, a periodic table will be provided.
-
2Find your element on the periodic table. The table orders elements by atomic number and separates them into three main groups: metals, non-metals, and metalloids (semi-metals). Further elemental groupings include alkali metals, halogens, and noble gases.[2]
- Using the group (columns) or period (rows) can make the element easier to locate on the table.
- You can also search the table for the symbol of the element if you don’t know any other properties.
Advertisement -
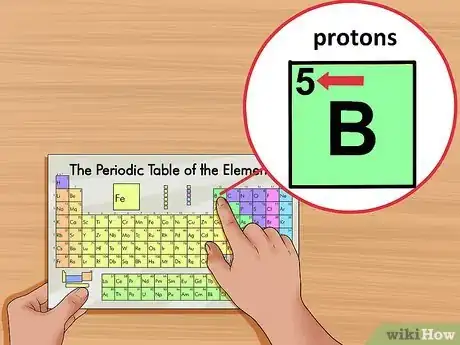
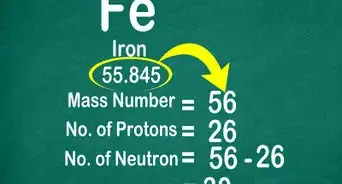
3Locate the element’s atomic number. The atomic number is located above the element symbol, in the upper left-hand corner of the square. The atomic number will tell you how many protons make up a single atom of an element.[3]
- For example, boron (B) has an atomic number of 5, therefore it has 5 protons.
-
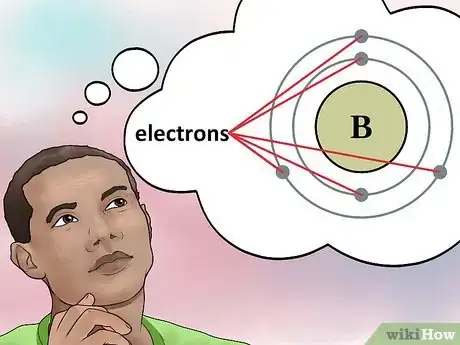
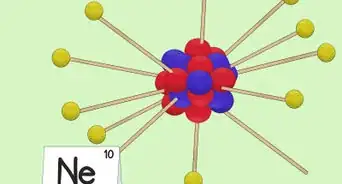
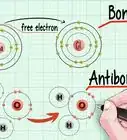
4Determine the number of electrons. Protons are particles in the nucleus of an atom that have a positive charge equal to +1. Electrons are particles that have a negative charge equal to -1. Therefore, an element in a neutral state will have the same number of protons and electrons.[4]
- For example, boron (B) has an atomic number of 5, therefore it has 5 protons and 5 electrons.
- However, if the element includes a negative or positive ion, then the protons and electrons will not be the same. You will have to calculate them. The ion number will appear as a small superscript after the element.
-
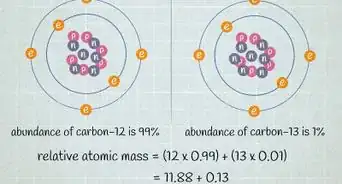
5Look for the atomic mass of the element. To find the number of neutrons, you will first need to find the atomic mass. An element’s atomic mass (also known as the atomic weight) is the weighted average mass of atoms of an element.[5] The atomic mass can be found underneath the symbol for the element.
- Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11.
-
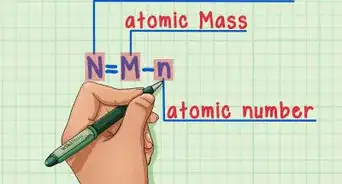
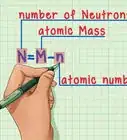
6Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass. Remember that the atomic number is the same as the number of protons, which you have already identified.[6]
- For our boron example, 11 (atomic mass) – 5 (atomic number) = 6 neutrons
Calculating the Electrons with Ions Present
-
1Identify the net charge. The net charge of an ion will appear as a small superscript number following the element. An ion is an atom that has a positive or negative charge due to the addition or removal of electrons.[7] Although the number of protons in the atom remains the same, the number of electrons is altered in an ion.
- Because an electron has a negative charge, when you remove electrons, the ion becomes positive. When you add more electrons, the ion becomes negative.
- For example, N3- has a -3 charge while Ca2+ has a +2 charge.
- Keep in mind that you do not have to do this calculation if there is no superscripted ion number following the element.
-
2Subtract the charge from the atomic number. When an ion has a positive charge, the atom has lost electrons. To calculate the remaining number of electrons, you subtract the amount of extra charge from the atomic number. In the case of a positive ion, there are more protons than electrons.[8]
- For example, Ca2+ has a +2 charge so it has lost 2 electrons from the neutral state. Calcium’s atomic number is 20, therefore the ion has 18 electrons.
-
3Add the charge to the atomic number for negative ions. When an ion has a negative charge, the atom has gained electrons. To calculate the total number of present electrons, you simply add the amount of extra charge to the atomic number. In the case of a negative ion, there are fewer protons than electrons.[9]
- For example, N3- has a -3 charge; therefore, it has gained 3 electrons compared to the neutral state. Nitrogen’s atomic number is 7, therefore this ion has 10 electrons.
Expert Q&A
-
QuestionHow do I find the number of protons when an atom has a -ve or +ve charge?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher The number of protons will never change. Atoms with negative or positive charges just indicate a gain or loss of electrons.
The number of protons will never change. Atoms with negative or positive charges just indicate a gain or loss of electrons. -
QuestionHow can I find the electron and proton numbers of actinium?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher You will find actinium in group 3, period 7 of the periodic table. The atomic number of actinium is 89, which means there are 89 protons. Because there is no net charge, we know that # protons = # electrons, so there are 89 electrons as well.
You will find actinium in group 3, period 7 of the periodic table. The atomic number of actinium is 89, which means there are 89 protons. Because there is no net charge, we know that # protons = # electrons, so there are 89 electrons as well. -
QuestionHow do I find the number of protons are in a nucleus when given the atomic mass?
 Community AnswerAll the protons are present in the nucleus, or centre of an atom. You need the atomic number to find the amount of protons and/or electrons, unless you have the amount of neutrons and the atomic mass, in which case you can simply subtract the amount of neutrons from the atomic mass, leaving the amount of protons in the atom. The atomic number (number at the top) is the amount of protons and the amount of electrons. So if an element has an atomic number of 5, you know that it has 5 protons and 5 electrons. The atomic mass (number at the bottom) is the amount of protons and neutrons added together. Whichever you know, you subtract from the atomic mass.
Community AnswerAll the protons are present in the nucleus, or centre of an atom. You need the atomic number to find the amount of protons and/or electrons, unless you have the amount of neutrons and the atomic mass, in which case you can simply subtract the amount of neutrons from the atomic mass, leaving the amount of protons in the atom. The atomic number (number at the top) is the amount of protons and the amount of electrons. So if an element has an atomic number of 5, you know that it has 5 protons and 5 electrons. The atomic mass (number at the bottom) is the amount of protons and neutrons added together. Whichever you know, you subtract from the atomic mass.
References
- ↑ http://periodic.lanl.gov/index.shtml
- ↑ http://edtech2.boisestate.edu/lindabennett1/502/Periodic%20Table%20e%20config/PTable_organized.html
- ↑ http://education.jlab.org/qa/pen_number.html
- ↑ https://chemistrytalk.org/protons-neutrons-electrons/
- ↑ https://chemistrytalk.org/protons-neutrons-electrons/
- ↑ http://education.jlab.org/qa/mathnuceus_01.html
- ↑ http://www.qrg.northwestern.edu/projects/vss/docs/propulsion/1-what-is-an-ion.html
- ↑ https://www.brooklyn.cuny.edu/bc/ahp/SDPS/SD.PS.ions.html
- ↑ https://sciencing.com/calculate-charge-ion-5955179.html
About This Article
The easiest way to find the number of protons, neutrons, and electrons for an element is to look at the element’s atomic number on the periodic table. That number is equal to the number of protons. The number of protons is equal to the number of electrons, unless there’s an ion superscript listed after the element. To find the number of neutrons, subtract the element’s atomic number from its atomic mass (the number listed underneath the element).