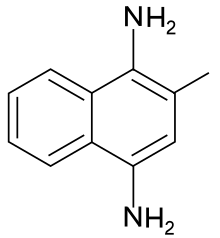
2-Methylnaphthalene-1,4-diamine
2-Methylnaphthalene-1,4-diamine is a synthetic menadione analog with vitamin K activity.[2][3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylnaphthalene-1,4-diamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H12N2 | |
| Molar mass | 172.231 g·mol−1 |
| Appearance | yellowish crystals[1] |
| Melting point | 110-113 °C[2][1] |
| dihydrochloride is freely soluble[3] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
2-Methylnaphthalene-1,4-diamine was first synthesized in 1925.[1][2] In 1942 two different research groups noted the vitamin K activity of the compound.[2][4][5] It forms a dihydrochloride salt (C11H14Cl2N2) with hydrochloric acid and one of the aforementioned research groups suggested the name vitamin K6 for the salt.[2]
2-Methylnaphthalene-1,4-diamine and its dihydrochloride can be made from 2-methylnaphthalene or its close analogs. Dihydrochloride blackens without melting at about 300 °C.[2]
4-Amino-3-methyl-1-naphthol or its dihydrochloride have not been used as commercial medicinal forms of vitamin K unlike phylloquinone and menadione for example.[6]
Oral toxicity of dihydrochloride for rats is approximately the same as for 4-amino-2-methyl-1-naphthol hydrochloride.[2]
References
- Veselý V, Kapp J (1925). "Sur les dérivés nitres du méthyl-2-naphtalène". Recueil des Travaux Chimiques des Pays-Bas. 44 (4): 360–375. doi:10.1002/recl.19250440409. ISSN 0165-0513.
- Veldstra H, Wiardi PW (1943). "Water soluble antihemorrhagic substances I: synthesis of 2-methyl-4-aminonaphthol-1 hydrochloride and of 2-methyl-1,4-diaminonaphthalene dihydrochloride, the water soluble synthetic vitamins K5 and K6". Recueil des Travaux Chimiques des Pays-Bas. 62 (2): 75–84. doi:10.1002/recl.19430620203. ISSN 0165-0513.
- Budavari S, et al. (2000). The Merck index (12th ed.). Chapman & Hall Electronic Pub. Division. p. 1581. ISBN 9781584881292.
- Baker BR, Carlson GH (1942). "Water-soluble compounds with antihemorrhagic activity". Journal of the American Chemical Society. 64 (11): 2657–2664. doi:10.1021/ja01263a038. ISSN 0002-7863.
- Robinson FA, Holland DO (1948). "Preparation of water-soluble derivatives of 2-methylnaphthalene". Journal of the Chemical Society. 2: 182–186. doi:10.1039/JR9480000182. ISSN 0368-1769. PMID 18906382.
- Fiore LD, et al. (2001). "Anaphylactoid reactions to vitamin K". Journal of Thrombosis and Thrombolysis. 11 (2): 175–183. doi:10.1023/A:1011237019082. ISSN 1573-742X. PMID 11406734. S2CID 975055.