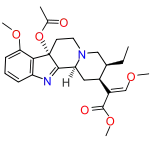
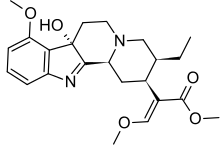
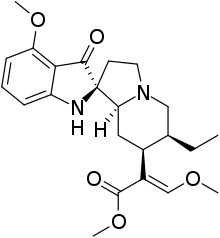
7-Hydroxymitragynine
7-Hydroxymitragynine is a terpenoid indole alkaloid from the plant Mitragyna speciosa, commonly known as kratom.[3] It is often referred to as ‘7-OH’. It was first described in 1994[4] and is a natural product derived from the mitragynine present in the kratom leaf. It is considered an oxidized derivative and active metabolite of mitragynine.[5] 7-OH binds to opioid receptors like mitragynine, but research suggests that 7-OH binds with greater potency[6] and contributes heavily to the analgesic activity of mitragynine as a metabolite.[7]
 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Methyl (2E)-2-[(2S,3S,7aS,12bS)-3-ethyl-7a-hydroxy-8-methoxy-1,2,3,4,6,7,7a,12b-octahydroindolo[2,3-a]quinolizin-2-yl]-3-methoxyprop-2-enoate | |
| Other names | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H30N2O5 | |
| Molar mass | 414.502 g·mol−1 |
| log P | 1.266 |
| Acidity (pKa) | 12.203 |
| Basicity (pKb) | 1.794 |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Metabolism
After a kratom study, it was revealed that 7-OH converts into mitragynine pseudoindoxyl.[8][9] Interestingly, this even more potent opioid was revealed to exist in a mixture of stereoisomers in biological systems.[10]
| Compound | Affinities (KiTooltip Inhibitor constant) | Ratio | Ref | ||
|---|---|---|---|---|---|
| MORTooltip μ-Opioid receptor | DORTooltip δ-Opioid receptor | KORTooltip κ-Opioid receptor | MOR:DOR:KOR | ||
| 7-Hydroxymitragynine | 13.5 | 155 | 123 | 1:11:9 | [11] |
| Mitragynine | 7.24 | 60.3 | 1,100 | 1:8:152 | [11] |
| Mitragynine pseudoindoxyl | 0.087 | 3.02 | 79.4 | 1:35:913 | [11] |
Pharmacology
7-Hydroxymitragynine, like mitragynine, appears to be a mixed opioid receptor agonist/antagonist, acting as a partial agonist at µ-opioid receptors and as a competitive antagonist at δ- and κ-opioid receptors.[12][13] It also acts on alpha 2 adrenergic and 5-HT2A receptors.[13] Evidence suggests that 7-OH is more potent than both mitragynine and morphine. 7-OH does not activate the β-arrestin pathway like traditional opioids, meaning symptoms such as respiratory depression, constipation and sedation are much less pronounced.[12]
7-OH is generated from mitragynine in vivo by hepatic metabolism and may account for a significant portion of the effects traditionally associated with mitragynine. Although 7-OH occurs naturally in kratom leaves, it does so in such low amounts that any ingested 7-OH is inconsequential compared to the 7-OH generated in the body.[12]
See also
- Ajmalicine
- Mitragynine
- Mitragynine pseudoindoxyl
- Mitraphylline
- β-Prodine - molecule overlaying 7-hydroxymitragynine's opioid QSAR (Quantitative structure-activity relationship)
References
- Chemical Abstracts Service: Columbus, OH, 2004; RN 174418-82-7 (accessed via SciFinder Scholar, version 2007.3; November 30, 2011)
- Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-15.
- Matsumoto K, Horie S, Ishikawa H, Takayama H, Aimi N, Ponglux D, Watanabe K (March 2004). "Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa". Life Sciences. 74 (17): 2143–2155. doi:10.1016/j.lfs.2003.09.054. PMID 14969718.
- Ponglux D, Wongseripipatana S, Takayama H, Kikuchi M, Kurihara M, Kitajima M, et al. (December 1994). "A New Indole Alkaloid, 7 alpha-Hydroxy-7H-mitragynine, from Mitragyna speciosa in Thailand". Planta Medica. 60 (6): 580–581. doi:10.1055/s-2006-959578. PMID 17236085.
- "7-Hydroxymitragynine - Green Leaf Kratom - Kratom Blogs Archives". Green Leaf Kratom. 2020-08-19. Retrieved 2020-08-22.
- Kruegel AC, Grundmann O (May 2018). "The medicinal chemistry and neuropharmacology of kratom: A preliminary discussion of a promising medicinal plant and analysis of its potential for abuse". Neuropharmacology. 134 (Pt A): 108–120. doi:10.1016/j.neuropharm.2017.08.026. PMID 28830758. S2CID 24009429.
- Spetea M, Schmidhammer H (June 2019). "Unveiling 7-Hydroxymitragynine as the Key Active Metabolite of Mitragynine and the Promise for Creating Novel Pain Relievers". ACS Central Science. 5 (6): 936–938. doi:10.1021/acscentsci.9b00462. PMC 6598155. PMID 31263752.
- Váradi A, Marrone GF, Palmer TC, Narayan A, Szabó MR, Le Rouzic V, et al. (September 2016). "Mitragynine/Corynantheidine Pseudoindoxyls As Opioid Analgesics with Mu Agonism and Delta Antagonism, Which Do Not Recruit β-Arrestin-2". Journal of Medicinal Chemistry. 59 (18): 8381–8397. doi:10.1021/acs.jmedchem.6b00748. PMC 5344672. PMID 27556704.
- Kamble SH, León F, King TI, Berthold EC, Lopera-Londoño C, Siva Rama Raju K, et al. (December 2020). "Metabolism of a Kratom Alkaloid Metabolite in Human Plasma Increases Its Opioid Potency and Efficacy". ACS Pharmacology & Translational Science. 3 (6): 1063–1068. doi:10.1021/acsptsci.0c00075. PMC 7737207. PMID 33344889.
- Kamble SH, León F, King TI, Berthold EC, Lopera-Londoño C, Siva Rama Raju K, et al. (December 2020). "Metabolism of a Kratom Alkaloid Metabolite in Human Plasma Increases Its Opioid Potency and Efficacy". ACS Pharmacology & Translational Science. 3 (6): 1063–1068. doi:10.1021/acsptsci.0c00075. PMC 7737207. PMID 33344889.
- Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, et al. (April 2002). "Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands". Journal of Medicinal Chemistry. 45 (9): 1949–1956. doi:10.1021/jm010576e. PMID 11960505.
- Eastlack SC, Cornett EM, Kaye AD (June 2020). "Kratom-Pharmacology, Clinical Implications, and Outlook: A Comprehensive Review". Pain and Therapy. 9 (1): 55–69. doi:10.1007/s40122-020-00151-x. PMC 7203303. PMID 31994019.
- Chang-Chien GC, Odonkor CA, Amorapanth P (2017). "Is Kratom the New 'Legal High' on the Block?: The Case of an Emerging Opioid Receptor Agonist with Substance Abuse Potential". Pain Physician. 20 (1): E195–E198. doi:10.36076/ppj.2017.1.E195. PMID 28072812.
Further reading
- Takayama H (August 2004). "Chemistry and pharmacology of analgesic indole alkaloids from the rubiaceous plant, Mitragyna speciosa". Chemical & Pharmaceutical Bulletin. 52 (8): 916–928. doi:10.1248/cpb.52.916. PMID 15304982.