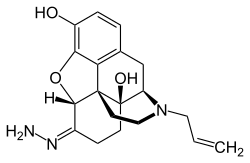
Naloxazone
Naloxazone is an irreversible μ-opioid receptor antagonist which is selective for the μ1 receptor subtype.[1] Naloxazone produces very long lasting antagonist effects as it forms a covalent bond to the active site of the μ-opioid receptor,[2] thus making it impossible for the molecule to unbind and blocking the receptor permanently until the receptor is recycled by endocytosis.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(5α)-17-Allyl-3,14-dihydroxy-4,5-epoxymorphinan-6-one hydrazone | |
| Other names
Naloxone- 6-hydrazone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
PubChem CID |
|
| |
| Properties | |
| C19H23N3O3 | |
| Molar mass | 341.40422 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Naloxazone is the hydrazone analog of naloxone. It has been reported that naloxazone is unstable in acidic solution, dimerizing into the more stable and much more potent antagonist naloxonazine via the free NH2 of the hydrazone to form an azine linkage.[3] Under conditions in which no naloxonazine formation could be detected, naloxazone did not display irreversible μ opioid receptor binding.[3]
See also
- Chlornaltrexamine, an irreversible mixed agonist-antagonist
- Oxymorphazone, an irreversible μ-opioid full agonist
References
- Pasternak, G.; Childers; Snyder, S. (1980). "Opiate analgesia: Evidence for mediation by a subpopulation of opiate receptors". Science. 208 (4443): 514–6. Bibcode:1980Sci...208..514P. doi:10.1126/science.6245448. PMID 6245448.
- Ling, Geoffrey S.F.; Simantov, Ronit; Clark, Janet A.; Pasternak, Gavril W. (1986). "Naloxonazine actions in vivo". European Journal of Pharmacology. 129 (1–2): 33–8. doi:10.1016/0014-2999(86)90333-X. PMID 3021478.
- Hahn, Elliot F.; Pasternak, Gavril W. (1982). "Naloxonazine, a potent, long-lasting inhibitor of opiate binding sites". Life Sciences. 31 (12–13): 1385–8. doi:10.1016/0024-3205(82)90387-3. PMID 6292633.