Acyl-CoA thioesterase 9
Acyl-CoA thioesterase 9 is a protein that is encoded by the human ACOT9 gene. It is a member of the acyl-CoA thioesterase superfamily, which is a group of enzymes that hydrolyze Coenzyme A esters. There is no known function, however it has been shown to act as a long-chain thioesterase at low concentrations, and a short-chain thioesterase at high concentrations.[5]
| ACOT9 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ACOT9, ACATE2, MT-ACT48, MTACT48, CGI-16, Acyl-CoA thioesterase 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 300862 MGI: 1928939 HomoloGene: 8206 GeneCards: ACOT9 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Gene
Locus
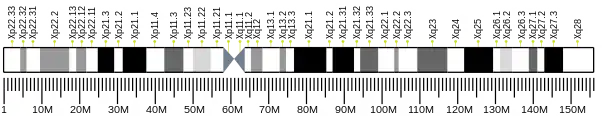
The ACOT9 gene is located at p22.11 on chromosome X. Located on the minus strand of the chromosome, the start is at 23,721,777 bp and the end is at 23,761,407 bp, which is a span of 39,631 base pairs.[6]
Function
The protein encoded by the ACOT9 gene is part of a family of Acyl-CoA thioesterases, which catalyze the hydrolysis of various Coenzyme A esters of various molecules to the free acid plus CoA. These enzymes have also been referred to in the literature as acyl-CoA hydrolases, acyl-CoA thioester hydrolases, and palmitoyl-CoA hydrolases. The reaction carried out by these enzymes is as follows:
CoA ester + H2O → free acid + coenzyme A
These enzymes use the same substrates as long-chain acyl-CoA synthetases, but have a unique purpose in that they generate the free acid and CoA, as opposed to long-chain acyl-CoA synthetases, which ligate fatty acids to CoA, to produce the CoA ester.[9] The role of the ACOT- family of enzymes is not well understood; however, it has been suggested that they play a crucial role in regulating the intracellular levels of CoA esters, Coenzyme A, and free fatty acids. Recent studies have shown that Acyl-CoA esters have many more functions than simply an energy source. These functions include allosteric regulation of enzymes such as acetyl-CoA carboxylase,[10] hexokinase IV,[11] and the citrate condensing enzyme. Long-chain acyl-CoAs also regulate opening of ATP-sensitive potassium channels and activation of Calcium ATPases, thereby regulating insulin secretion.[12] A number of other cellular events are also mediated via acyl-CoAs, for example signal transduction through protein kinase C, inhibition of retinoic acid-induced apoptosis, and involvement in budding and fusion of the endomembrane system.[13][14][15] Acyl-CoAs also mediate protein targeting to various membranes and regulation of G Protein α subunits, because they are substrates for protein acylation.[16] In the mitochondria, acyl-CoA esters are involved in the acylation of mitochondrial NAD+ dependent dehydrogenases; because these enzymes are responsible for amino acid catabolism, this acylation renders the whole process inactive. This mechanism may provide metabolic crosstalk and act to regulate the NADH/NAD+ ratio in order to maintain optimal mitochondrial beta oxidation of fatty acids.[17] The role of CoA esters in lipid metabolism and numerous other intracellular processes are well defined, and thus it is hypothesized that ACOT- enzymes play a role in modulating the processes these metabolites are involved in.[18]
Homology/Evolution
_vs._Time_(MYA)_in_ACOT9.png.webp)
Orthologs
There are many orthologs of ACOT9, the house mouse (Mus musculus) being one of the most similar, where the ACOT9 gene is found at 72.38cM on chromosome X.[19] The range of orthologs extends to mammals, birds, amphibians, anamorphic fungi, and others.
| Sequence number | Genus and species | Common name | Date of divergence (MYA) | Accession number | Sequence length | Sequence identity | Sequence similarity | Notes |
|---|---|---|---|---|---|---|---|---|
| 1 | Homo sapiens | Human | 0 | NP_001028755.2 | 439 | 100% | 100% | Human |
| 2 | Mus musculus | House mouse | 91 | NP_062710.2 | 439 | 83% | 90% | Rodent |
| 3 | Pteropus alecto | Black flying fox | 97.4 | XP_006911668.1 | 480 | 81% | 91% | Bat |
| 4 | Gallus gallus | Chicken | 324.5 | NP_001012841.1 | 425 | 69% | 87% | Bird |
| 5 | Pseudopodoces humilis | Ground tit | 324.5 | XP_005516751.1 | 417 | 68% | 85% | Bird |
| 6 | Columba livia | Rock dove | 324.5 | XP_005503782.1 | 402 | 67% | 86% | Bird |
| 7 | Geospiza fortis | Medium ground finch | 324.5 | XP_005424946.1 | 417 | 67% | 85% | Bird |
| 8 | Pelodiscus sinensis | Chinese soft shelled turtle | 324.5 | XP_006112565.1 | 439 | 67% | 85% | Reptile |
| 9 | Xenopus tropicalis | Western clawed frog | 361.2 | AAI61600.1 | 418 | 65% | 82% | Amphibian |
| 10 | Danio rerio | Zebrafish | 454.6 | AAI59216.1 | 434 | 60% | 80% | Fish |
| 11 | Ceratitis capitata | Mediterranean fruit fly | 910 | JAB97119.1 | 433 | 32% | 58% | Insect |
| 12 | Glarea lozoyensis 74030 | Anamorphic fungus | 1368 | EHL00310.1 | 350 | 24% | 47% | Fungus |
Paralogs
In mice, which is one of the closest orthologs, ACOT10 is a known paralog of the ACOT9 gene.[20]
Expression
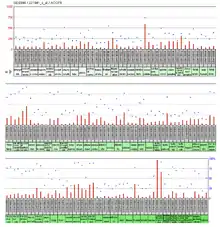
Expression of the ACOT9 is ubiquitous throughout the tissues in humans. Tissues with a value of over 500 in the large-scale analysis of the human transcriptome were the globus pallidus and colorectal adenocarcinoma.[21] The expressed sequence tag (or EST) abundance profile also shows ubiquitous/near ubiquitous, expression throughout human tissues.[22]
Transcription factors
There are numerous transcription factors throughout the ACOT9 promoter sequence. Some of the notable factors are heat shock factors and transcription factor II B (TFIIB) recognition elements.
| Transcription factor | Start | End | Strand | Sequence |
|---|---|---|---|---|
| X gene core promoter element 1 | 683 | 693 | - | ggGCGGgaccg |
| Doublesex and mab-3 related transcription factor 1 | 81 | 101 | + | tttttttgagacaTTGTctcc |
| cAMP-responsive element binding protein 1 | 491 | 511 | - | agggcgTGACgtcgagaagag |
| Sp4 transcription factor | 660 | 676 | - | ccagggGGCGtggccgc |
| Stimulating protein 1, ubiquitous zinc finger transcription factor | 682 | 698 | - | tccggGGGCgggaccgc |
| Heat shock factor 1 | 24 | 48 | + | caggactaaactAGAAtctccagcc |
| E2F transcription factor 2 | 808 | 824 | + | ccatcGCGCgcacggca |
| Nuclear factor of activated T-cells 5 | 380 | 398 | + | tttGGAAagttgcccagga |
| ZF5 POZ domain zinc finger, zinc finger protein 161 (secondary DNA binding preference) | 811 | 825 | + | tcgCGCGcacggcag |
| B-cell-specific activator protein | 678 | 706 | - | cagcggtgtccgggGGCGggaccgcggcg |
| Pax-6 paired domain binding site | 54 | 72 | + | gtctcAAGCatcagttttt |
| ZF5 POZ domain zinc finger, zinc finger protein 161 (secondary DNA binding preference) | 651 | 665 | - | ggcCGCGctgtgccg |
| Pax-6 paired domain binding site | 758 | 776 | + | ttttaTCGCctcagtttcc |
| Mammalian C-type LTR TATA box | 751 | 767 | - | ggcgaTAAAagacgcac |
| Nuclear factor Y (Y-box binding factor) | 624 | 638 | + | cccgCCAAtgaacgg |
| Transcription factor II B (TFIIB) recognition element | 356 | 362 | + | ccgCGCC |
| Transcription factor II B (TFIIB) recognition element | 440 | 446 | - | ccgCGCC |
| Transcription factor II B (TFIIB) recognition element | 734 | 740 | - | ccgCGCC |
| Nuclear factor Y (Y-box binding factor) | 581 | 595 | - | ccacTCAAtcagttg |
| CCAAT/enhancer binding protein alpha | 529 | 543 | - | tcggttgaGTAAacg |
Secondary structure
There are two regions in the ACOT9 gene sequence that are labeled as BFIT (Brown Fat Inducible Thioesterase) and BACH (Brain Acyl CoA Hydrolase) regions. These regions are part of a hotdog fold superfamily, which has been found to be used in a variety of cell roles.[23] Predictions show there to be various alpha-helices throughout the structure,[24] suggesting it is a transmembrane protein.
Interactions
A mitochondrial cleavage site can be found at amino acid 30 in the ACOT9 sequence, and the probability of export to the mitochondria is 0.9374.[25] The Acyl-CoA thioesterase 9 protein is estimated to be 60.9% mitochondrial, 21.7% cytoplasmic, 8.7% nuclear, 4.3% in the plasma membrane, and 4.3% in the endoplasmic reticulum.[26]
The ACOT9 protein has been found to interact with the following proteins either experimentally or through co-expression:[27]
References
- GRCh38: Ensembl release 89: ENSG00000123130 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000025287 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Tillander V, Arvidsson Nordström E, Reilly J, Strozyk M, Van Veldhoven PP, Hunt MC, Alexson SE (Mar 2014). "Acyl-CoA thioesterase 9 (ACOT9) in mouse may provide a novel link between fatty acid and amino acid metabolism in mitochondria". Cellular and Molecular Life Sciences. 71 (5): 933–48. doi:10.1007/s00018-013-1422-1. hdl:10616/41794. PMID 23864032. S2CID 18767370.
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D (June 12, 2002). "Human Feb. 2009 (GRCh37/hg19) Assembly". The human genome browser at UCSC. UCSC Genome Bioinformatics. Retrieved March 12, 2014.
- Gu J, MacHugh DE, McGivney BA, Park SD, Katz LM, Hill EW (Nov 2010). "Association of sequence variants in CKM (creatine kinase, muscle) and COX4I2 (cytochrome c oxidase, subunit 4, isoform 2) genes with racing performance in Thoroughbred horses". Equine Veterinary Journal. Supplement. 42 (38): 569–75. doi:10.1111/j.2042-3306.2010.00181.x. PMID 21059062.
- Poupon V, Bègue B, Gagnon J, Dautry-Varsat A, Cerf-Bensussan N, Benmerah A (Jul 1999). "Molecular cloning and characterization of MT-ACT48, a novel mitochondrial acyl-CoA thioesterase". The Journal of Biological Chemistry. 274 (27): 19188–94. doi:10.1074/jbc.274.27.19188. PMID 10383425.
- Mashek DG, Bornfeldt KE, Coleman RA, Berger J, Bernlohr DA, Black P, DiRusso CC, Farber SA, Guo W, Hashimoto N, Khodiyar V, Kuypers FA, Maltais LJ, Nebert DW, Renieri A, Schaffer JE, Stahl A, Watkins PA, Vasiliou V, Yamamoto TT (Oct 2004). "Revised nomenclature for the mammalian long-chain acyl-CoA synthetase gene family". Journal of Lipid Research. 45 (10): 1958–61. doi:10.1194/jlr.e400002-jlr200. PMID 15292367.
- Ogiwara H, Tanabe T, Nikawa J, Numa S (Aug 1978). "Inhibition of rat-liver acetyl-coenzyme-A carboxylase by palmitoyl-coenzyme A. Formation of equimolar enzyme-inhibitor complex". European Journal of Biochemistry. 89 (1): 33–41. doi:10.1111/j.1432-1033.1978.tb20893.x. PMID 29756.
- Srere PA (Dec 1965). "Palmityl-coenzyme A inhibition of the citrate-condensing enzyme". Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 106 (3): 445–55. doi:10.1016/0005-2760(65)90061-5. PMID 5881327.
- Gribble FM, Proks P, Corkey BE, Ashcroft FM (Oct 1998). "Mechanism of cloned ATP-sensitive potassium channel activation by oleoyl-CoA". The Journal of Biological Chemistry. 273 (41): 26383–7. doi:10.1074/jbc.273.41.26383. PMID 9756869.
- Nishizuka Y (Apr 1995). "Protein kinase C and lipid signaling for sustained cellular responses". FASEB Journal. 9 (7): 484–96. doi:10.1096/fasebj.9.7.7737456. PMID 7737456. S2CID 31065063.
- Glick BS, Rothman JE (1987). "Possible role for fatty acyl-coenzyme A in intracellular protein transport". Nature. 326 (6110): 309–12. Bibcode:1987Natur.326..309G. doi:10.1038/326309a0. PMID 3821906. S2CID 4306469.
- Wan YJ, Cai Y, Cowan C, Magee TR (Jun 2000). "Fatty acyl-CoAs inhibit retinoic acid-induced apoptosis in Hep3B cells". Cancer Letters. 154 (1): 19–27. doi:10.1016/s0304-3835(00)00341-4. PMID 10799735.
- Duncan JA, Gilman AG (Jun 1998). "A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein alpha subunits and p21(RAS)". The Journal of Biological Chemistry. 273 (25): 15830–7. doi:10.1074/jbc.273.25.15830. PMID 9624183.
- Berthiaume L, Deichaite I, Peseckis S, Resh MD (Mar 1994). "Regulation of enzymatic activity by active site fatty acylation. A new role for long chain fatty acid acylation of proteins". The Journal of Biological Chemistry. 269 (9): 6498–505. doi:10.1016/S0021-9258(17)37399-4. PMID 8120000.
- Hunt MC, Alexson SE (Mar 2002). "The role Acyl-CoA thioesterases play in mediating intracellular lipid metabolism". Progress in Lipid Research. 41 (2): 99–130. doi:10.1016/s0163-7827(01)00017-0. PMID 11755680.
- "ACOT9 gene detail". Mouse Genome Database. Retrieved 2014-06-19.
- "Gene: Acot9". Ensembl release 75.
- "Large-scale analysis of the human transcriptome (HG-U133A)". National Center for Biotechnology Information. Retrieved 10 May 2014.
- "EST Profile Hs.298885 - ACOT9: Acyl-CoA thioesterase 9". Retrieved 10 May 2014.
- Dillon SC, Bateman A (Aug 2004). "The Hotdog fold: wrapping up a superfamily of thioesterases and dehydratases". BMC Bioinformatics. 5: 109. doi:10.1186/1471-2105-5-109. PMC 516016. PMID 15307895.
- "SDSC Biology WorkBench 3.2 Pele Program".
- Claros MG, Vincens P (Nov 1996). "Computational method to predict mitochondrially imported proteins and their targeting sequences". European Journal of Biochemistry. 241 (3): 779–86. doi:10.1111/j.1432-1033.1996.00779.x. PMID 8944766.
- "PSORTII Prediction Tool".
- Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, Bork P, von Mering C (Jan 2009). "STRING 8--a global view on proteins and their functional interactions in 630 organisms". Nucleic Acids Research. 37 (Database issue): D412–6. doi:10.1093/nar/gkn760. PMC 2686466. PMID 18940858.
External links
- Human ACOT9 genome location and ACOT9 gene details page in the UCSC Genome Browser.
Further reading
- Mulkearns EE, Cooper JA (Apr 2012). "FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis". Molecular Biology of the Cell. 23 (7): 1330–42. doi:10.1091/mbc.E11-09-0812. PMC 3315808. PMID 22323290.
- Hunt MC, Yamada J, Maltais LJ, Wright MW, Podesta EJ, Alexson SE (Sep 2005). "A revised nomenclature for mammalian acyl-CoA thioesterases/hydrolases". Journal of Lipid Research. 46 (9): 2029–32. doi:10.1194/jlr.E500003-JLR200. PMID 16103133.
- Alkhaja AK, Jans DC, Nikolov M, Vukotic M, Lytovchenko O, Ludewig F, Schliebs W, Riedel D, Urlaub H, Jakobs S, Deckers M (Jan 2012). "MINOS1 is a conserved component of mitofilin complexes and required for mitochondrial function and cristae organization" (PDF). Molecular Biology of the Cell. 23 (2): 247–57. doi:10.1091/mbc.E11-09-0774. PMC 3258170. PMID 22114354.
- Poupon V, Bègue B, Gagnon J, Dautry-Varsat A, Cerf-Bensussan N, Benmerah A (Jul 1999). "Molecular cloning and characterization of MT-ACT48, a novel mitochondrial acyl-CoA thioesterase". The Journal of Biological Chemistry. 274 (27): 19188–94. doi:10.1074/jbc.274.27.19188. PMID 10383425.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.