Ammonium orthomolybdate
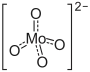
Ammonium orthomolybdate is the inorganic compound with the chemical formula (NH4)2MoO4. It is a white solid that is prepared by treating molybdenum trioxide with aqueous ammonia. Upon heating these solutions, ammonia is lost, to give ammonium heptamolybdate ((NH4)6Mo7O24·4H2O).
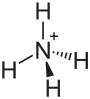  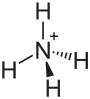 | |
| Names | |
|---|---|
| Other names
Diammonium molybdate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.032.741 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (NH4)2MoO4 | |
| Molar mass | 196.02 g/mol |
| Appearance | colorless monoclinic crystals |
| Density | 2.276 g/cm3 |
| Melting point | decomposes upon heating |
| soluble | |
| Solubility | insoluble in alcohol and liquid ammonia |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1870 mg/kg (rabbit, oral) 2200 mg/kg (guinea pig, oral) 1600 mg/kg (cat, oral)[1] |
LDLo (lowest published) |
120 mg Mo/kg (rat, oral) 120 mg Mo/kg (guinea pig, oral)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Uses
Ammonium orthomolybdate is used as a corrosion inhibitor and is an intermediate in some schemes to win molybdenum from its ores.[2] It is also used for decorating ceramics, and for colorimetric analysis of phosphates and arsenates.
Chemical reactions
Heating ammonium orthomolybdate solid or treatment with acid gives molybdenum trioxide. Such reactions proceed via ammonium dimolybdate. This equilibrium is exploited in the purification of molybdenum from its ores. Aqueous solutions of ammonium orthomolybdate react with hydrogen sulfide to give ammonium tetrathiomolybdate:
- (NH4)2MoO4 + 4 H2S → (NH4)2MoS4 + 4 H2O
It reacts with arsenic acid upon heating to form a canary yellow precipitate of ammonium α-Keggin molybdoarsenate.
- (NH4)2MoO4 + H3AsO4 → (NH4)2[As(Mo3O10)4] + 21 NH4NO3 + 12 H2O[3]
References
- "Molybdenum (soluble compounds, as Mo)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Roger F. Sebenik et al. "Molybdenum and Molybdenum Compounds" in Ullmann's Encyclopedia of Chemical Technology 2005; Wiley-VCH, Weinheim. doi:10.1002/14356007.a16_655
- Murakami, Masahiko; Hirano, Masaya; Shibahara, Toshiaki; Kubota, Toshio (18 February 2016). "Speciation of Inorganic Arsenic in Groundwater as Molybdoarsenate by On-Site Solid-Phase Extraction and Graphite Furnace Atomic Absorption Spectrometry". Analytical Letters. 49 (13): 2119–2131. doi:10.1080/00032719.2015.1135932. S2CID 100305249.