Biosafety level
A biosafety level (BSL), or pathogen/protection level, is a set of biocontainment precautions required to isolate dangerous biological agents in an enclosed laboratory facility. The levels of containment range from the lowest biosafety level 1 (BSL-1) to the highest at level 4 (BSL-4). In the United States, the Centers for Disease Control and Prevention (CDC) have specified these levels in a publication referred to as BMBL.[2] In the European Union, the same biosafety levels are defined in a directive.[3] In Canada the four levels are known as Containment Levels.[4] Facilities with these designations are also sometimes given as P1 through P4 (for pathogen or protection level), as in the term P3 laboratory.[5]
At the lowest level of biosafety, precautions may consist of regular hand-washing and minimal protective equipment. At higher biosafety levels, precautions may include airflow systems, multiple containment rooms, sealed containers, positive pressure personnel suits, established protocols for all procedures, extensive personnel training, and high levels of security to control access to the facility. Health Canada reports that world-wide until 1999 there were recorded over 5,000 cases of accidental laboratory infections and 190 deaths.[6]
History
The first prototype Class III (maximum containment) biosafety cabinet was fashioned in 1943 by Hubert Kaempf Jr., then a U.S. Army soldier, under the direction of Arnold G. Wedum, Director (1944–1969) of Industrial Health and Safety at the United States Army Biological Warfare Laboratories, Camp Detrick, Maryland. Kaempf was tired of his MP duties at Detrick and was able to transfer to the sheet metal department working with the contractor, the H.K. Ferguson Co.[7]
On 18 April 1955, fourteen representatives met at Camp Detrick in Frederick, Maryland. The meeting was to share knowledge and experiences regarding biosafety, chemical, radiological, and industrial safety issues that were common to the operations at the three principal biological warfare (BW) laboratories of the U.S. Army.[8] Because of the potential implication of the work conducted at biological warfare laboratories, the conferences were restricted to top level security clearances. Beginning in 1957, these conferences were planned to include non-classified sessions as well as classified sessions to enable broader sharing of biological safety information. It was not until 1964, however, that conferences were held in a government installation not associated with a biological warfare program.[9]
Over the next ten years, the biological safety conferences grew to include representatives from all federal agencies that sponsored or conducted research with pathogenic microorganisms. By 1966, it began to include representatives from universities, private laboratories, hospitals, and industrial complexes. Throughout the 1970s, participation in the conferences continued to expand and by 1983 discussions began regarding the creation of a formal organization.[9] The American Biological Safety Association (ABSA) was officially established in 1984 and a constitution and bylaws were drafted the same year. As of 2008, ABSA includes some 1,600 members in its professional association.[9]
In 1977, Jim Peacock of the Australian Academy of Science asked Bill Snowdon, then chief of the CSIRO's Australian Animal Health Laboratory (AAHL) if he could have the newly released United States' National Institutes of Health and the British equivalent requirements for the development of infrastructure for bio-containment reviewed by AAHL personnel with a view to recommending the adoption of one of them by Australian authorities. The review was carried out by CSIRO AAHL Project Manager Bill Curnow and CSIRO Engineer Arthur Jenkins. They drafted outcomes for each of the levels of security. AAHL was notionally classified as "substantially beyond P4". These were adopted by the Australian Academy of Science and became the basis for Australian legislation. It opened in 1985 costing $185 million, built on Corio Oval.[10] The Australian Animal Health Laboratory is a Class 4/ P4 Laboratory.[11][12]
In 2003, the Chinese Academy of Sciences approved the construction of mainland China's first BSL-4 laboratory at the Wuhan Institute of Virology (WIV). In 2014, the WIV's National Bio-safety Laboratory was built at a cost of 300 million yuan (US$44 million), in collaboration and with assistance from the French government's CIRI lab.[13][14][15]
In 2007 a scientific review paper stated that the Canadian Science Centre for Human and Animal Health, which was designed in the early 1990s, "has become the prototype for modern BSL4 laboratories".
Starting with the 2020 Covid-19 pandemic near the facilities of the WIV, work in biocontainment facilities has been politicized, especially in the US Senate for example as the result of Rand Paul's work.[16] Russia asked questions on 25 October 2022 in the United Nations over the presence in Ukraine of biolabs.[17] In the month of April 2023 the descent into civil war of Sudan caused worries at the World Health Organization over its National Public Laboratory as contending factions battled over its area and one kicked out the NPL staff and installed a military base in its premises. At the time the facility contained organisms rated at BSL2.[18][19]
Levels
Biosafety level 1
Biosafety level 1 (BSL-1) is suitable for work with well-characterized agents which do not cause disease in healthy humans. In general, these agents should pose minimal potential hazard to laboratory personnel and the environment.[20] At this level, precautions are limited relative to other levels. Laboratory personnel must wash their hands upon entering and exiting the lab. Research with these agents may be performed on standard open laboratory benches without the use of special containment equipment. However, eating and drinking are generally prohibited in laboratory areas.[20] Potentially infectious material must be decontaminated before disposal, either by adding a chemical such as bleach or isopropanol or by packaging for decontamination elsewhere.[20] Personal protective equipment is only required for circumstances where personnel might be exposed to hazardous material.[20] BSL-1 laboratories must have a door which can be closed to limit access to the lab. However, it is not necessary for BSL-1 labs to be isolated from the general building.[21]
This level of biosafety is appropriate for work with several kinds of microorganisms including non-pathogenic strains of Escherichia coli and Staphylococcus, Bacillus subtilis, Saccharomyces cerevisiae and other organisms not suspected to contribute to human disease.[22] Due to the relative ease and safety of maintaining a BSL-1 laboratory, these are the types of laboratories generally used as teaching spaces for high schools and colleges.[21]
Biosafety level 2
At this level, all precautions used at Biosafety Level 1 are followed, and some additional precautions are taken. BSL-2 differs from BSL-1 in that:
- "laboratory personnel have specific training in handling pathogenic agents and are directed by competent scientists."[23][24]
- Access to the laboratory is limited when work is being conducted.
- Certain procedures in which infectious aerosols or splashes may be created are conducted in biological safety cabinets or other physical containment equipment.[20]
- Extreme precautions are taken with contaminated sharp items.
Biosafety level 2 is suitable for work involving agents of moderate potential hazard to personnel and the environment.[21] This includes various microbes that cause mild disease to humans, or are difficult to contract via aerosol in a lab setting.[25] Examples of pathogens classified as "Risk Group 2" in the United States include hepatitis A, B, and C viruses, human immunodeficiency virus (HIV), pathogenic strains of Escherichia coli and Staphylococcus, Salmonella, Plasmodium falciparum, and Toxoplasma gondii.[25][26] Notably, the European Union departs from the United States and classifies HIV and hepatitis B – G as Risk Group 3 agents best handled at BSL-3.[27]
Prions, the infectious agents that transmit prion diseases such as vCJD, are typically handled under Biosafety Level 2 or higher.[23] This is due to the lack of any evidence of aerosol transmission and relatively higher infective dose of prion diseases, though some circumstances (such as handling animal-infective prions in a facility which cares for vulnerable animals) would require BSL-3 conditions.[23]
Biosafety level 3

Biosafety level 3 is appropriate for work involving microbes which can cause serious and potentially lethal disease via the inhalation route.[20] This type of work can be done in clinical, diagnostic, teaching, research, or production facilities.[21] Here, the precautions undertaken in BSL-1 and BSL-2 labs are followed, as well as additional measures including:
- A laboratory-specific biosafety manual must be drafted which details how the laboratory will operate in compliance with all safety requirements.[20]
- All laboratory personnel are provided medical surveillance and offered relevant immunizations (where available) to reduce the risk of an accidental or unnoticed infection.[20]
- All procedures involving infectious material must be done within a biological safety cabinet.[20]
- Laboratory personnel must wear solid-front protective clothing (i.e. gowns that tie in the back). This cannot be worn outside of the laboratory and must be discarded or decontaminated after each use.[20]
In addition, the facility which houses the BSL-3 laboratory must have certain features to ensure appropriate containment. The entrance to the laboratory must be separated from areas of the building with unrestricted traffic flow.[20] Additionally, the laboratory must be behind two sets of self-closing doors (to reduce the risk of aerosols escaping).[21] The construction of the laboratory is such that it can be easily cleaned. Carpets are not permitted, and any seams in the floors, walls, and ceilings are sealed to allow for easy cleaning and decontamination.[20] Additionally, windows must be sealed, and a ventilation system installed which forces air to flow from the "clean" areas of the lab to the areas where infectious agents are handled.[20] Air from the laboratory must be filtered before it can be recirculated.[20]
A 2015 study by USA Today journalists identified more than 200 lab sites in the U.S. that were accredited biosafety levels 3 or 4.[28] The Proceedings of a Workshop on "Developing Norms for the Provision of Biological Laboratories in Low-Resource Contexts" provides a list of BSL-3 laboratories in those countries.[29]
Biosafety level 3 is commonly used for research and diagnostic work involving various microbes which can be transmitted by aerosols and/or cause severe disease. These include Francisella tularensis, Mycobacterium tuberculosis, Chlamydia psittaci, Venezuelan equine encephalitis virus, Eastern equine encephalitis virus, SARS-CoV-1, MERS-CoV, Coxiella burnetii, Rift Valley fever virus, Rickettsia rickettsii, several species of Brucella, chikungunya, yellow fever virus, West Nile virus, Yersinia pestis,[26] and SARS-CoV-2.[30]
Biosafety level 4

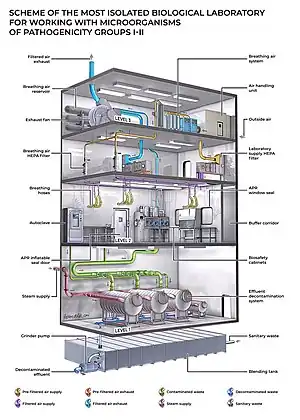
Biosafety level 4 (BSL-4) is the highest level of biosafety precautions, and is appropriate for work with agents that could easily be aerosol-transmitted within the laboratory and cause severe to fatal disease in humans for which there are no available vaccines or treatments. BSL-4 laboratories are generally set up to be either cabinet laboratories or protective-suit laboratories. In cabinet laboratories, all work must be done within a class III biosafety cabinet. Materials leaving the cabinet must be decontaminated by passing through an autoclave or a tank of disinfectant. The cabinets themselves are required to have seamless edges to allow for easy cleaning. Additionally, the cabinet and all materials within must be free of sharp edges to reduce the risk of damage to the gloves. In a protective-suit laboratory, all work must be done in a class II biosafety cabinet by personnel wearing a positive pressure suit. To exit the BSL-4 laboratory, personnel must pass through a chemical shower for decontamination, then a room for removing the positive-pressure suit, followed by a personal shower. Entry into the BSL-4 laboratory is restricted to trained and authorized individuals, and all persons entering and exiting the laboratory must be recorded.[20]
As with BSL-3 laboratories, BSL-4 laboratories must be separated from areas that receive unrestricted traffic. Additionally, airflow is tightly controlled to ensure that air always flows from "clean" areas of the lab to areas where work with infectious agents is being performed. The entrance to the BSL-4 lab must also employ airlocks to minimize the possibility that aerosols from the lab could be removed from the lab. All laboratory waste, including filtered air, water, and trash must also be decontaminated before it can leave the facility.[20]
Biosafety level 4 laboratories are used for diagnostic work and research on easily transmitted pathogens which can cause fatal disease. These include a number of viruses known to cause viral hemorrhagic fever such as Marburg virus, Ebola virus, Lassa virus, and Crimean-Congo hemorrhagic fever. Other pathogens handled at BSL-4 include Hendra virus, Nipah virus, and some flaviviruses. Additionally, poorly characterized pathogens which appear closely related to dangerous pathogens are often handled at this level until sufficient data are obtained either to confirm continued work at this level, or to permit working with them at a lower level.[26] This level is also used for work with Variola virus, the causative agent of smallpox, though this work is only performed at the Centers for Disease Control and Prevention in Atlanta, United States, and the State Research Center of Virology and Biotechnology in Koltsovo, Russia.[31]
 Regular inspection of positive-pressure suits to locate any leaks[32]
Regular inspection of positive-pressure suits to locate any leaks[32]
 The circular containment tube separates the patient table in the "hot" zone (pathogen present) from the "cold" zone around this MRI machine.
The circular containment tube separates the patient table in the "hot" zone (pathogen present) from the "cold" zone around this MRI machine._door.jpg.webp) Air pressure resistant (APR) door to separate the hot and cold zones
Air pressure resistant (APR) door to separate the hot and cold zones Working inside a BSL-4 lab with air hoses providing positive air pressure
Working inside a BSL-4 lab with air hoses providing positive air pressure Inside a Class III biological safety cabinet with an aerosol control platform
Inside a Class III biological safety cabinet with an aerosol control platform Effluent decontamination system of a BSL-4 lab of NIAID
Effluent decontamination system of a BSL-4 lab of NIAID
BSL-4 facilities for extraterrestrial samples
Sample-return missions that bring back to Earth samples obtained from a Category V body must be curated at facilities rated BSL-4. Because the existing BSL-4 facilities in the world do not provide the level of cleanliness necessary to such pristine samples,[33] there is a need to design a facility dedicated to curation of restricted (potentially biohazardous) extraterrestrial materials. The systems of such facilities must be able to contain unknown biohazards, as the sizes of any putative alien microorganisms are unknown. Ideally, it should filter particles down to 10 nanometers, and release of a particle 50 nanometers or larger is unacceptable under any circumstance.[34]
Because NASA and ESA are collaborating on the Mars Sample Return campaign, due to return samples from Mars in the early 2030s, the need for a Sample Receiving Facility (SRF) is becoming more pressing. An SRF is expected to take 7 to 10 years from design to completion,[35][36] and an additional two years is recommended for the staff to become proficient and accustomed to the facilities.[35]
List of BSL-4 facilities
According to a U.S. Government Accountability Office (GAO) report published on 4 October 2007, a total of 1,356 CDC/USDA registered BSL-3 facilities were identified throughout the United States.[37] Approximately 36% of these laboratories are located in academia. 15 BSL-4 facilities were identified in the U.S. in 2007, including nine at federal labs.[37] As of May 2021, there are 42 BSL-4 facilities in operation around the world, with a further 17 planned or under construction.[38]
The following is a list of existing BSL-4 facilities worldwide.
| Country | Location | Name | Date established |
Description |
|---|---|---|---|---|
| Argentina | Buenos Aires | National Service of Healthcare and Agriculture Quality (SENASA) | Diagnostic laboratory for foot-and-mouth disease.[39] | |
| Australia | Geelong, Victoria | Australian Centre for Disease Preparedness | 1985 | Capable of housing from large experimental animals to insects under conditions that exceed all BSL 4 requirements. The antecedent of all such facilities developed since the 1980s. Arguably the most researched design and construction project ever. The ACDP is subdivided into a number of isolation zones that can be managed at differing containment levels concurrently. CSIRO AAHL Project Manager and Architect, William Curnow, provided technical reviews to Canadian, Indian, UK and French Authorities and consulted with Dr Jerry Callis [PIADC] to UN FAO on matters of bio-containment. Formerly known as the Australian Animal Health Laboratory (AAHL) and renamed to Australian Centre for Disease Preparedness April 2020 |
| Melbourne | University of Melbourne – Doherty Institute for Infection and Immunity | 2014 | Diagnostic reference lab.[40][12] | |
| National High Security Laboratory | Operates under the auspice of the Victoria Infectious Diseases Reference Laboratory.[41] | |||
| Belarus | Minsk | Republican Research and Practical Center for Epidemiology and Microbiology (RPPCM) | Formerly the SRIEM.[42] | |
| Brazil | Pedro Leopoldo, Minas Gerais | Laboratório Nacional Agropecuário de Minas Gerais (Lanagro/MG) | 2014 | Focus on Agropecuary diseases and diagnostics, like the foot-and-mouth disease.[43] |
| Campinas, São Paulo | Laboratório Nacional de Máxima Contenção Biológica (LNMCB) | 2026 (expected) | It was announced in 2021 to be built near the Sincrotron lab.[44][45] | |
| Canada | Winnipeg, Manitoba | National Microbiology Laboratory | 1999[46] | Located at the Canadian Science Centre for Human and Animal Health, it is jointly operated by the Public Health Agency of Canada and the Canadian Food Inspection Agency.[47] |
| China | Wuhan, Hubei | Wuhan Institute of Virology of the Chinese Academy of Sciences | 2015 | Wuhan Institute of Virology has existed since 1956 and already hosted BSL-3 laboratories. A BSL-4 facility was completed in 2015, and became the first BSL-4 laboratory in China.[48] |
| Harbin, Heilongjiang | Harbin Veterinary Research Institute of the Chinese Academy of Agricultural Sciences | 2018 | Harbin Veterinary Research Institute researches prevention and control of major infectious diseases. China's second, and the first for large animals, BSL-4 lab.[49] | |
| Czech Republic | Těchonín, Pardubice Region | Biological Defense Center | 1971, rebuilt 2003–2007 | Hospital and research facility. Located at the Centrum biologické ochrany (Biological Defense Center). Operated by Army of the Czech Republic.[50] |
| France | Brétigny-sur-Orge, Essonne | French Armed Biomedical Research Institute, French Defence Health Service | French Army laboratory.[51] | |
| Lyon, Metropolis of Lyon | Jean Mérieux BSL-4 Laboratory | 1999 | Built and owned by the Fondation Mérieux. Since 2004, operated by INSERM.[52] | |
| Vert-le-Petit, Essonne | Laboratoire de la DGA | 2013 | Operated by the Ministry of Defense.[53] | |
| Gabon | Franceville, Haut-Ogooué Province | Centre International de Recherches Médicales de Franceville | This facility is operated by a research organization supported by both Gabonese (mainly) and French governments, and is West Africa's only P4 lab (BSL-4).[54] | |
| Germany | Berlin | Robert Koch Institute | 2015 | Diagnostic and experimental lab facility.[55] |
| Hamburg | Bernhard Nocht Institute for Tropical Medicine | 2014 | Part of the Leibniz Center Infection. National reference lab for tropical viruses.[56] | |
| Isle of Riems, Greifswald, Mecklenburg-Vorpommern | Friedrich Loeffler Institute | 2010 | Focus on animal viral diseases and diagnostics.[57] | |
| Marburg, Hesse | Philipps University of Marburg | 2008 | Focuses on hemorrhagic fever viruses.[58] | |
| Hungary | Budapest | National Center for Epidemiology | 1998 | Division of Virology operates three WHO National Reference Laboratories. The BSL-4 biosafety laboratory provides a modern means to process dangerous imported zoonotic viral pathogens.[59] |
| Pécs | University of Pécs | 2016 | Opened in 2016, part of Szentágothai János Kutatóközpont.[60] | |
| India | Bhopal, Madhya Pradesh | National Institute of High Security Animal Disease | 1998 | This facility deals especially to zoonotic organisms and emerging infectious disease threats.[61] |
| Hyderabad, Telangana | Centre for Cellular and Molecular Biology | 2009 | National BSL-4 Containment Facility for Human Infectious Diseases.[62] | |
| Pune, Maharashtra | National Institute of Virology | 2012 | India's most advanced BSL-4 category lab.[63] | |
| Italy | Rome, Lazio | Istituto Nazionale per le Malattie Infettive | 1997 | The "National Institute of Infectious Diseases" used to operate within the Lazzaro Spallanzani hospital; the facility is now independent and is home to five BSL-3 labs as well as a single BSL-4 laboratory, which was completed in 1997.[64] |
| Milan, Lombardy | Ospedale Luigi Sacco | 2006 | ||
| Japan | Musashimurayama, Tokyo | National Institute for Infectious Diseases | 2015 | Located at National Institute for Infectious Diseases, Department of Virology I. Built in 1981; operated at BSL-3 until 2015 due to opposition from nearby residents.[65] |
| Tsukuba, Ibaraki Prefecture | Institute of Physical and Chemical Research (RIKEN) | 1984 | Facility completed in 1984 but not operated as BSL-4 due to local opposition.[66] | |
| Philippines | New Clark City, Capas, Tarlac | Virology Institute of the Philippines | 2024 (expected) | First BSL-4 Lab in the Philippines when completed.[67] |
| Russia | Sergiyev Posad, Moscow Oblast | 48th Central Scientific Research Institute Sergiev Posad[42] | ||
| Koltsovo, Novosibirsk Oblast | State Research Center of Virology and Biotechnology (VECTOR) | One of two WHO-approved facilities for work on variola virus (AKA smallpox).[31] | ||
| Singapore | Singapore | DSO National Laboratories | End-2025 (expected) | First BSL-4 Lab in Singapore when completed.[68] |
| South Africa | Johannesburg, Gauteng | National Institute for Communicable Diseases | 2002 | [69] |
| South Korea | Cheongju, North Chungcheong Province | Korea Centers for Disease Control and Prevention | 2017 | First BSL-4 Lab in South Korea.[70][71] |
| Sweden | Solna, Stockholm County | Public Health Agency of Sweden | 2001 | The only BSL-4 facility in the Nordic region. Constructed for research and diagnostics of hemorrhagic fever viruses.[72] |
| Switzerland | Geneva, Canton of Geneva | University Hospital of Geneva | "Glove box" type laboratory; primarily for handling clinical samples.[73] | |
| Spiez, Canton of Bern | Spiez Laboratory | 2013 | Run by the Federal Office for Civil Protection of the Federal Department of Defence, Civil Protection and Sports.[74] | |
| Mittelhäusern, Canton of Bern | The Institute of Virology and Immunology IVI[75] | Part of the Food Safety and Veterinary Office (FSVO).[76] Primary purpose is diagnostics of highly pathogenic viruses.[74] | ||
| Republic of China (Taiwan) | National Defense University | Institute of Preventive Medicine | 1983 | [77] |
| Taipei, Taiwan | Kwen-yang Laboratory | |||
| United Kingdom | Camden, Greater London | Francis Crick Institute | 2015 | Has BSL-4 space but does not work on human pathogens.[78] |
| Colindale, Greater London | Public Health England's Centre for Infections | Department of Health laboratory. Diagnostics for various viral diseases.[79] Part of the European Network of Biosafety-Level-4 Laboratories.[80] | ||
| Mill Hill, Greater London | National Institute for Medical Research | Medical Research Council laboratory. Research and diagnostics for highly pathogenic viruses. Closed in 2017 and work moved to the Francis Crick Institute. Site demolished in 2018.[79] | ||
| Potters Bar, Hertfordshire | National Institute for Biological Standards and Control | Department of Health and Home Office laboratory. Develop assays and reagents for research on virulent pathogens.[79] | ||
| Addlestone, Surrey | Animal and Plant Health Agency | Department for Environment, Food and Rural Affairs laboratory. Diagnostics and research for animal diseases.[79] | ||
| Pirbright, Surrey | Institute for Animal Health | Biotechnology and Biological Sciences Research Council laboratory. Research on highly pathogenic animal diseases.[79] | ||
| Merial Animal Health | Private lab. Produces vaccines against foot and mouth disease and bluetongue disease.[79] | |||
| Porton Down, Wiltshire | Centre for Emergency Preparedness and Response | Department of Health laboratory. Diagnostics and research for haemorrhagic fever viruses.[79] Part of the European Network of Biosafety-Level-4 Laboratories.[80] | ||
| Defence Science and Technology Laboratory | Ministry of Defence laboratory. Focuses on protection from biological weapons.[79] | |||
| United States | Atlanta, Georgia | Centers for Disease Control and Prevention | Currently operates in two buildings. One of two facilities in the world that officially hold smallpox.[31] | |
| Georgia State University | 1997 | Research focus on B virus.[81] | ||
| Manhattan, Kansas | National Bio and Agro-Defense Facility (NBAF), Kansas State University | 2022 (expected) | Under construction. Facility to be operated by the Department of Homeland Security, and replace the Plum Island Animal Disease Center. Expected to be operational by 2022–2023.[82] | |
| Bethesda, Maryland | National Institutes of Health (NIH) | Located on the NIH Campus, it currently only operates with BSL-3 agents.[83] | ||
| Fort Detrick, Maryland | Integrated Research Facility | Operated by National Institute of Allergy and Infectious Diseases (NIAID). Focuses on animal models of human diseases.[84] | ||
| National Biodefense Analysis and Countermeasures Center | Operated by the Department of Homeland Security. Focus on potential bioterrorism threats.[85] | |||
| US Army Medical Research Institute of Infectious Diseases (USAMRIID) | 1969 | Run by the U.S. Army. Research focuses on biological threats to the U.S. military.[86][87] | ||
| Boston, Massachusetts | National Emerging Infectious Diseases Laboratory (NEIDL), Boston University | Built 2008, Opened 2012,[88] BSL-4 Approval in 2017[89] | Focus on potential threats to public health.[90] Operated by Boston University School of Medicine.[91] | |
| Hamilton, Montana | Rocky Mountain Laboratories Integrated Research Facility | 2008 | NIAID laboratory. Focus on vector-borne diseases.[92] | |
| Galveston, Texas | Galveston National Laboratory, National Biocontainment Facility | Opened in 2008, facility is operated by the University of Texas Medical Branch (UTMB).[93] | ||
| Shope Laboratory | 2004 | Operated by UTMB.[94] | ||
| San Antonio, Texas | Texas Biomedical Research Institute | 1999 | The only privately owned BSL-4 lab in the US.[95] | |
| Richmond, Virginia | Virginia Division of Consolidated Laboratories | 2003 | A BSL-4 lab that also acts as a BSL-3 lab.[96] |
Safety concerns
A North Carolina Mosquito & Vector Control Association (NCMVCA) study highlighted safety concerns. In the United States, laboratories can be funded by federal, state, private, non-profit, or academically. The last accounts for 72% of the funding.[97]
High-containment labs that are registered with the Centers for Disease Control and Prevention (CDC) and the U.S. Department of Agriculture's (USDA) Select Agent Program must adhere to Department of Defense standards.[98] Since BSL3 and 4 laboratories in the United States are regulated by either the CDC or USDA or another federal agency (depending on the pathogens they handle), no single federal agency is responsible for regulating or tracking the number of these labs.[99] U.S. high-containment laboratories that handle pathogens which are declared as "select agents" must be inspected periodically by the CDC or USDA, adhere to certain standards, and maintain ongoing education on biosecurity and biosafety policies as mandated by law.[100][101]
See also
References
- "Integrated Research Facility". niaid.nih.gov. NIAID. Archived from the original on 28 November 2014. Retrieved 14 November 2014.
- Chosewood LC, Wilson DE, eds. (2009). Biosafety in Microbiological and Biomedical Laboratories (5th ed.). Centers for Disease Control and Prevention. ISBN 978-0-1608-5042-4. Retrieved 1 April 2020.
- Directive 2000/54/EC of the European Parliament and of the Council of 18 September 2000 on the protection of workers from risks related to exposure to biological agents at work (seventh individual directive within the meaning of Article 16(1) of Directive 89/391/EEC)
- Canada, Public Health Agency of. "Chapter 2: The Laboratory Biosafety Guidelines: 3rd Edition 2004 – Biological safety – Canada.ca". www.canada.ca. Archived from the original on 23 February 2018. Retrieved 7 May 2018.
- Laboratory Safety Monograph: A Supplement to the NIH Guidelines for Recombinant DNA Research. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, National Cancer Institute, Office of Research Safety. 1978. passim.
- "Biosafety at Ryerson" (PDF). Ryerson University Facilities Management and Design. Archived from the original (PDF) on 16 February 2021. Retrieved 4 February 2021.
- Covt, Norman M. (1997), "A History of Fort Detrick, Maryland" Archived 2008-09-22 at the Wayback Machine, 3rd edition. Kaempf retired from Fort Detrick in 1994, having completed more than 50 years service. He was chief of the mechanical branch, Directorate of Engineering and Housing.
- Manuel S. Barbeito; Richard H. Kruse. "A History of the American Biological Safety Association". American Biological Safety Association. Archived from the original on 20 June 2008. Retrieved 14 August 2008.
- "American Biological Safety Association Collection : NAL Collections : National Agricultural Library". United States Department of Agriculture: National Agricultural Library. 11 February 2009. Archived from the original on 27 February 2009. Retrieved 11 February 2009.
- "CSIRO: Geelong – Australian Animal Health Laboratory".
- Lowenthal, John (May 2016). "Overview of the CSIRO Australian Animal Health Laboratory". Journal of Infection and Public Health. 9 (3): 236–239. doi:10.1016/j.jiph.2016.04.007. PMC 7102798. PMID 27118215.
- Racaniello V (14 July 2014). "Visiting biosafety level-4 laboratories". Virology Blog. Archived from the original on 18 April 2016. Retrieved 3 April 2016.
- "Inside the Wuhan lab: French engineering, deadly viruses and a big mystery". Washington Post.
- Cyranoski, David (23 February 2017). "Inside the Chinese lab poised to study world's most dangerous pathogens". Nature. 542 (7642): 399–400. Bibcode:2017Natur.542..399C. doi:10.1038/nature.2017.21487. PMID 28230144.
- "China Inaugurates the First Biocontainment Level 4 Laboratory in Wuhan". Wuhan Institute of Virology, Chinese Academy of Sciences. 3 February 2015. Archived from the original on 3 March 2016. Retrieved 9 April 2016.
- "JUST IN: Rand Paul Asks Samantha Power: 'Did USAID Fund Coronavirus Research In Wuhan China?'". Forbes Breaking News. YouTube. 26 April 2023.
- LEDERER, EDITH M. (25 October 2022). "Russia seeks UN probe of claims on Ukraine biological labs". The Associated Press.
- Wallace, Danielle (25 April 2023). "WHO official warns of 'high risk of biological hazard' in Sudan after fighters seize laboratory: reports". FOX News Network, LLC.
- Horton, Jake (26 April 2023). "Sudan crisis: WHO warns of biological hazard at seized lab". BBC.
- "Section IV-Laboratory Biosafety Level Criteria". Biosafety in Microbiological and Biomedical Laboratories, 5th ed (PDF). U.S. Department of Health and Human Services. December 2009. pp. 30–59. Archived (PDF) from the original on 9 April 2016. Retrieved 2 April 2016.
- Richmond JY. "The 1, 2, 3's of Biosafety Levels" (PDF). Archived (PDF) from the original on 19 March 2015. Retrieved 2 April 2016.
- "Health & Safety Manual – Biological Safety". Columbia University Environmental Health and Safety. Archived from the original on 27 March 2016. Retrieved 2 April 2016.
- "Section VIII-H: Prion Diseases". Biosafety in Microbiological and Biomedical Laboratories (PDF). U.S. Department of Health and Human Services. June 2020. Retrieved 3 August 2021.
...Prion Diseases...In the laboratory setting, prions from human tissue and human prions propagated in animals can be manipulated at BSL-2 or higher
- "Principles and Concepts of Biosafety | Environmental Health & Safety | University of Missouri". ehs.missouri.edu. Retrieved 25 January 2023.
- "Section III-Principles of Biosafety". Biosafety in Microbiological and Biomedical Laboratories, 5th ed (PDF). U.S. Department of Health and Human Services. December 2009. pp. 22–28. Archived (PDF) from the original on 10 March 2016. Retrieved 9 April 2016.
- For a list of infectious agents and the recommended biosafety level at which they should be studied, see "Section VIII-Agent Summary Statements". Biosafety in Microbiological and Biomedical Laboratories, 5th ed. U.S. Department of Health and Human Services. December 2009. pp. 123–289. Archived (PDF) from the original on 27 March 2016. Retrieved 9 April 2016.
- European Parliament (4 August 2021). "Directive 2000/54/EC – biological agents at work | Safety and health at work EU-OSHA". Osha.europa.eu. ISSN 0378-6978. Retrieved 12 March 2023.
- Penzenstadler, Nick (28 May 2015). "State incidents highlight bioterror lab concerns". Post Crescent. USA Today Network.
- "APPENDIX E LIST OF LABS IDENTIFIED IN LOW-RESOURCE COUNTRIES". National Academy of Sciences. 2019. Retrieved 4 February 2021.
- "Interim Laboratory Biosafety Guidelines for Handling and Processing Specimens Associated with Coronavirus Disease 2019 (COVID-19)". Coronavirus Disease 2019 (COVID-19) Lab Biosafety Guidelines. Centers for Disease Control and Prevention. 11 February 2020. Retrieved 1 April 2020.
- "Section VIII-Agent Summary Statements". Biosafety in Microbiological and Biomedical Laboratories, 5th ed (PDF). U.S. Department of Health and Human Services. December 2009. p. 219. Archived (PDF) from the original on 13 May 2016. Retrieved 4 May 2016.
- Seligson, Susan (7 March 2013). "Video Offers Glimpse of Biosafety Level 4 Lab Science webcast "threads the NEIDL"". BU Today. Archived from the original on 10 December 2014. Retrieved 5 December 2014.
- How to Protect Mars Samples on Earth. Jeremy Hsu, Space.com. 3 December 2009.
- "European Science Foundation – Mars Sample Return backward contamination – Strategic advice and requirements" (PDF). Archived from the original (PDF) on 2 June 2016.
- "7: "Sample-Receiving Facility and Program Oversight"". Assessment of Planetary Protection Requirements for Mars Sample Return Missions (Report). National Research Council. 2009. p. 59.
- Mars Sample Return: Issues and Recommendations (Planetary Protection Office Summary) Task Group on Issues in Sample Return. National Academies Press, Washington, DC (1997)
- "High-Containment Biosafety Laboratories: Preliminary Observations on the Oversight of the Proliferation of BSL-3 and BSL-4 Laboratories in the United States" (PDF). United States Government Accountability Office. 4 October 2007. Archived (PDF) from the original on 12 February 2016. Retrieved 26 May 2016.
- Lentzos, Filippa; Koblentz, Gregory D. (May 2021). "Mapping Maximum Biological Containment Labs Globally" (PDF). Archived from the original (PDF) on 1 November 2022. Retrieved 3 November 2022.
- "Risk Analysis:Risk of Importing Foot-and-Mouth Disease in Susceptible Species and Products from a region of Patagonia, Argentina" (PDF). U.S. Department of Agriculture, National Import Export Services, Veterinary Services. January 2014. pp. 60–62. Archived (PDF) from the original on 21 October 2016. Retrieved 3 April 2016.
- "Members: The Doherty Institute for Infection and Immunity". Global Virus Network. May 2013. Archived from the original on 20 March 2016. Retrieved 3 April 2016.
- "Laboratories: High Security/Quarantine". Victorian Infectious Diseases Reference Laboratory. Archived from the original on 19 April 2016. Retrieved 8 April 2016.
- Kuhn, Jens H.; Bao, Yiming; Bavari, Sina; Becker, Stephan; Bradfute, Steven; Brister, J. Rodney; Bukreyev, Alexander A.; Caì, Yíngyún; Chandran, Kartik; Davey, Robert A.; Dolnik, Olga; Dye, John M.; Enterlein, Sven; Gonzalez, Jean-Paul; Formenty, Pierre; Freiberg, Alexander N.; Hensley, Lisa E.; Honko, Anna N.; Ignatyev, Georgy M.; Jahrling, Peter B.; Johnson, Karl M.; Klenk, Hans-Dieter; Kobinger, Gary; Lackemeyer, Matthew G.; Leroy, Eric M.; Lever, Mark S.; Lofts, Loreen L.; Mühlberger, Elke; Netesov, Sergey V.; Olinger, Gene G.; Palacios, Gustavo; Patterson, Jean L.; Paweska, Janusz T.; Pitt, Louise; Radoshitzky, Sheli R.; Ryabchikova, Elena I.; Saphire, Erica Ollmann; Shestopalov, Aleksandr M.; Smither, Sophie J.; Sullivan, Nancy J.; Swanepoel, Robert; Takada, Ayato; Towner, Jonathan S.; van der Groen, Guido; Volchkov, Viktor E.; Wahl-Jensen, Victoria; Warren, Travis K.; Warfield, Kelly L.; Weidmann, Manfred; Nichol, Stuart T. (June 2013). "Virus nomenclature below the species level: A standardized nomenclature for laboratory animal-adapted strains and variants of viruses assigned to the family Filoviridae". Archives of Virology. 158 (6): 1425–1432. doi:10.1007/s00705-012-1594-2. ISSN 0304-8608. PMC 3669655. PMID 23358612. Retrieved 16 June 2021.
- "Lanagro/MG é o primeiro do Brasil com nível de biossegurança máximo". MAPA – Ministério da Agricultura, Pecuária e Abastecimento. August 2014. Archived from the original on 23 February 2018. Retrieved 22 February 2018.
- "Abre ao Orçamento Fiscal da União, em favor dos Ministérios da Ciência, Tecnologia e Inovações e da Justiça e Segurança Pública". 14.242, Act of 19 November 2021 (in Portuguese).
- "Ministro da Ciência confirma construção de laboratório de biossegurança 4 junto ao Sirius". G1 (in Brazilian Portuguese). 17 May 2021. Retrieved 12 May 2022.
- "Canadian Science Centre for Human and Animal Health Celebrates 20 Years of Scientific Excellence" (Press release). Winnipeg, MB: Government of Canada. Public Health Agency of Canada. 2 May 2019. Archived from the original on 11 January 2022. Retrieved 12 March 2023.
- "National Microbiology Laboratory (NML) Overview". Public Health Agency of Canada. Archived from the original on 21 March 2016. Retrieved 8 April 2016.
- "China Inaugurates the First Biocontainment Level 4 Laboratory in Wuhan". Wuhan Institute of Virology, Chinese Academy of Sciences. 3 February 2015. Archived from the original on 3 March 2016. Retrieved 9 April 2016.
- "China launches high-level biosafety lab". Xinhua. 8 August 2018. Archived from the original on 14 October 2018. Retrieved 13 March 2019.
- "Biological Defence Department at Techonin". Ministry of Defense & Armed Forces of the Czech Republic. Archived from the original on 26 April 2016. Retrieved 9 April 2016.
- "Un laboratoire militaire hautement sécurisé à Brétigny en 2015". Le Parisien (in French). 20 May 2014. Retrieved 5 March 2020.
- "Jean Mérieux BSL-4 Laboratory". Fondation Mérieux. Archived from the original on 6 May 2016. Retrieved 11 April 2016.
- "Inauguration du laboratoire biologique P4 de la DGA" (in French). Ministére de la Défense. Archived from the original on 8 May 2016. Retrieved 11 April 2016.
- "Centre International de Recherches Medicales de Franceville" (in French). CIRMF. Archived from the original on 15 October 2014. Retrieved 30 September 2014.
- "Das Hochsicherheitslabor im Robert Koch-Institut". Robert Koch Institut. Archived from the original on 19 May 2016. Retrieved 16 April 2016.
- "Bernhard Nocht Institute for Tropical Medicine (BNI)". Heinrich Pette Institute. Archived from the original on 27 April 2016. Retrieved 16 April 2016.
- "Friedrich Loeffler Institute, Germany". Caverion. Archived from the original on 22 April 2016. Retrieved 16 April 2016.
- "Philipps-University Marburg". Philipps-University Marburg. Archived from the original on 11 June 2016. Retrieved 16 April 2016.
- "Division of Virology". Országos Epidemiológiai Központ. Archived from the original on 24 September 2013. Retrieved 16 April 2016.
- "BSL-4 Laboratory - Virology research group | University of Pécs". szkk.pte.hu.
- "Bio-containment Laboratory". National Institute of High Security Animal Diseases, India. Archived from the original on 19 March 2016. Retrieved 20 April 2016.
- "Stone laid for stem cell research lab in Hyderabad". The Hindu. Archived from the original on 16 January 2016. Retrieved 25 April 2016.
- "NIV Prune lab gets BSL-4". The Hindu. Archived from the original on 16 August 2017. Retrieved 24 April 2016.
- "Storia dell'Istituto" (in Italian). IRCCS Lazzaro Spallanzani. Archived from the original on 7 March 2014. Retrieved 1 May 2016.
- "Deadly disease lab opens amid local fears". Japan Times. 15 October 2015. Archived from the original on 28 April 2016. Retrieved 1 May 2016.
- "Bio lab handling highly dangerous agents to open in suburban Tokyo". Japan Bullet. 3 August 2015. Archived from the original on 15 March 2018. Retrieved 14 March 2018.
- Domingo, Katrina (25 May 2021). "Virology Institute of the Philippines to rise in Tarlac in 2 years: DOST". ABS-CBN News. Retrieved 26 May 2021.
- "DSO biosafety lab to get S$90 million upgrade to handle more lethal and infectious viruses". DSO National Laboratories. Retrieved 4 March 2021.
- "South Africa National Institute for Communicable Diseases". African National Public Health Institutes. Archived from the original on 18 March 2016. Retrieved 4 May 2016.
- Da-sol, Kim (16 March 2017). "Korea to open first deadly virus biosafety laboratory". The Korea Herald. Retrieved 16 June 2021.
- Kim, Il-Hwan; Jang, Jun Hyeong; Jo, Su-Kyoung; No, Jin Sun; Seo, Seung-Hee; Kim, Jun-Young; Jung, Sang-Oun; Kim, Jeong-Min; Lee, Sang-Eun; Park, Hye-Kyung; Kim, Eun-Jin; Jeon, Jun Ho; Choi, Myung-Min; Ryu, Boyeong; Jang, Yoon Suk; Kim, Hwami; Lee, Jin; Shin, Seung-Hwan; Kim, Hee Kyoung; Kim, Eun-Kyoung; Park, Ye Eun; Yoo, Cheon-Kwon; Lee, Sang-Won; Han, Myung-Guk; Rhie, Gi-Eun; Kang, Byung Hak (October 2020). "2019 Tabletop Exercise for Laboratory Diagnosis and Analyses of Unknown Disease Outbreaks by the Korea Centers for Disease Control and Prevention". Osong Public Health and Research Perspectives. 11 (5): 280–285. doi:10.24171/j.phrp.2020.11.5.03. ISSN 2210-9099. PMC 7577389. PMID 33117632.
- "P4-laboratoriet vid Folkhälsomyndigheten" (in Swedish). Public Health Agency of Sweden. 25 August 2015. Archived from the original on 13 March 2020. Retrieved 8 July 2020.
- Cherpillod, P. "Management of suspect viral hemorrhagic fever patient in Geneva". Schweizerische Union fur Labormedizin. Archived from the original on 16 August 2016. Retrieved 10 May 2016.
- "Convention on the Prohibition of the Development, Production and Stockpiling of Bacteriological (Biological) and Toxin Weapons and on their Destruction" (PDF). Switzerland Federal Department of Defence, Civil Protection, and Sports. 2016. Archived (PDF) from the original on 3 June 2016. Retrieved 10 May 2016.
- "Homepage".
- "Federal Food Safety and Veterinary Office".
- "Case of SARS reported in a laboratory research worker in Taiwan". Weekly Releases (1997–2007). Eurosurveillance. 7 (51). 18 December 2003. doi:10.2807/esw.07.51.02347-en. Archived from the original on 11 June 2016. Retrieved 18 May 2016.
- Ewen Callaway (6 June 2013). "London biomedical hub sets its research agenda". Nature. doi:10.1038/nature.2013.13143. S2CID 180705493. Archived from the original on 7 June 2016. Retrieved 26 May 2016.
- Davison N; Lentzos F (2012). "E8: High-Containment Laboratories-UK Case Study". Biosecurity Challenges of the Global Expansion of High-Containment Biological Laboratories. National Academies Press. pp. 176–177. ISBN 978-0-309-22575-5. Archived from the original on 23 June 2016. Retrieved 26 May 2016.
- Nisii, Carla; Castilletti, Concetta; Raoul, Hervé; Hewson, Roger; Brown, David; Gopal, Robin; Eickmann, Markus; Gunther, Stephan; Mirazimi, Ali; Koivula, Tuija; Feldmann, Heinz; Di Caro, Antonino; Capobianchi, Maria R.; Ippolito, Giuseppe (2013). "Biosafety Level-4 Laboratories in Europe: Opportunities for Public Health, Diagnostics, and Research". PLOS Pathogens. 9 (1): e1003105. doi:10.1371/journal.ppat.1003105. PMC 3547859. PMID 23349630.
- "Operating a BSL-4 Laboratory in a University Setting". Tradeline. 16 December 2003. Archived from the original on 30 June 2016. Retrieved 28 May 2016.
- "Leveraging the National Bio and Agro-defense Facility". Kansas State University. Archived from the original on 10 June 2016. Retrieved 28 May 2016.
- "An Integrated Research Facility: Questions and Answers". National Institute of Allergy and Infectious Diseases. Archived from the original on 22 June 2016. Retrieved 28 May 2016.
- "Integrated Research Facility Overview". National Institute of Allergy and Infectious Disease. Archived from the original on 5 July 2016. Retrieved 28 May 2016.
- "National Biodefense Analysis and Countermeasures Center". Department of Homeland Security. 6 July 2009. Archived from the original on 20 May 2016. Retrieved 28 May 2016.
- "USAMRIID: Biodefense Solutions to Protect our Nation". U.S. Army Medical Department. Archived from the original on 5 June 2016. Retrieved 28 May 2016.
- "USAMRIID Biological Safety". U.S. Army Medical Department. Archived from the original on 18 May 2016. Retrieved 28 May 2016.
- "NEIDL Goes Public: BU Biosafety Labs Offer Tours to Press, Politicians". Retrieved 30 December 2018.
- "NEIDL BSL-4 Lab Gets Green Light". Retrieved 30 December 2018.
- "National Emerging Infectious Diseases Laboratories: About – Mission and Safety". Boston University. Archived from the original on 4 June 2016. Retrieved 28 May 2016.
- "About | National Emerging Infectious Diseases Laboratories". www.bu.edu. Retrieved 29 December 2022.
- "Rocky Mountain Labs Overview". National Institute for Allergy and Infectious Disease. Archived from the original on 29 April 2016. Retrieved 28 May 2016.
- "Galveston National Laboratory Fact Sheet". Archived from the original on 5 October 2014. Retrieved 30 September 2014.
- "Center for Biodefense and Emerging Infectious Diseases: Safety and Biocontainment". UTMB Health. Archived from the original on 5 January 2016. Retrieved 28 May 2016.
- "About Texas Biomed: Biosafety Level 4 Laboratory". Texas Biomedical Research Institute. Archived from the original on 3 April 2016. Retrieved 3 April 2016.
- "State of the art laboratory opens in Richmond". Virginia Commonwealth University News. Archived from the original on 8 August 2014. Retrieved 1 June 2021.
- NCMVCA study Archived 2017-01-31 at the Wayback Machine- Retrieved 2017-01-19
- DoD Safety Standards for Microbiological and Biomedical Laboratories Archived 2017-01-25 at the Wayback Machine- Retrieved 2017-01-19
- GAO publication Archived 2017-01-20 at the Wayback Machine- Retrieved 2017-01-19
- "Select Agent – an overview ScienceDirect Topics". www.sciencedirect.com. Retrieved 14 June 2021.
- "Select Agent Program". ors.od.nih.gov. Retrieved 14 June 2021.