Momelotinib
 | |
| Names | |
|---|---|
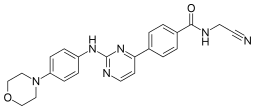
| Preferred IUPAC name
N-(Cyanomethyl)-4-{2-[4-(morpholin-4-yl)anilino]pyrimidin-4-yl}benzamide | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank |
|
| KEGG | |
PubChem CID |
|
| UNII |
|
| |
| |
| Properties | |
| C23H22N6O2 | |
| Molar mass | 414.469 g·mol−1 |
| Pharmacology | |
| None | |
| By mouth | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| Clinical data | |
|---|---|
| Other names | Momelotinib hydrochloride hydrate (JAN JP), Momelotinib dihydrochloride (USAN US) |
| License data |
|
| Identifiers | |
| PDB ligand | |
Momelotinib, sold under the brand name Ojjaara, is an anticancer medication used for the treatment of myelofibrosis.[1] It is a Janus kinase inhibitor and it is taken by mouth.[1]
The most common adverse reactions include dizziness, fatigue, bacterial infection, hemorrhage, thrombocytopenia, diarrhea, and nausea.[2]
Momelotinib was approved for medical use in the United States in September 2023.[1][2][3]
Medical uses
Momelotinib is indicated for the treatment of intermediate or high-risk myelofibrosis in adults with anemia.[1][2][3]
Pharmacology
References
- "Ojjaara- momelotinib tablet". DailyMed. 15 September 2023. Retrieved 20 September 2023.
- "FDA Roundup: September 19, 2023". U.S. Food and Drug Administration (FDA) (Press release). 19 September 2023. Retrieved 20 September 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Novel Drug Approvals for 2023". U.S. Food and Drug Administration (FDA). 15 September 2023. Retrieved 20 September 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Pardanani A, Lasho T, Smith G, Burns CJ, Fantino E, Tefferi A (August 2009). "CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients". Leukemia. 23 (8): 1441–5. doi:10.1038/leu.2009.50. PMID 19295546.
External links
- Clinical trial number NCT04173494 for "A Study of Momelotinib Versus Danazol in Symptomatic and Anemic Myelofibrosis Patients (MOMENTUM)" at ClinicalTrials.gov
- Clinical trial number NCT01969838 for "Momelotinib Versus Ruxolitinib in Subjects With Myelofibrosis (Simplify 1)" at ClinicalTrials.gov
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.