Deucravacitinib
Deucravacitinib, sold under the brand name Sotyktu, is medication used for the treatment of moderate-to-severe plaque psoriasis.[5] It is a tyrosine kinase 2 (TYK2) inhibitor and it is taken by mouth.[5] It was developed by Bristol Myers Squibb.[7]
 | |
| Clinical data | |
|---|---|
| Pronunciation | /duːˌkrævəˈsɪtɪnɪb/ doo-KRA-və-SI-ti-nib |
| Trade names | Sotyktu |
| Other names | BMS-986165 |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 99% |
| Protein binding | 82–90% |
| Metabolism | Liver (primarily CYP1A2) |
| Metabolites | BMT-153261 (active) |
| Elimination half-life | 10 hours |
| Excretion | Feces, urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.329.069 |
| Chemical and physical data | |
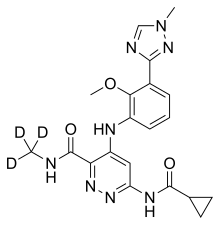
| Formula | C20H19D3N8O3 |
| Molar mass | 425.466 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Deucravacitinib was approved for medical use in the United States in September 2022,[5][8] and in Australia in December 2022.[1] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication.[9]
Medical uses
Deucravacitinib is indicated for the treatment of adults with moderate-to-severe plaque psoriasis.[5]
Mechanism of action
It acts as a highly selective allosteric inhibitor of non-receptor tyrosine-protein kinase 2 (TYK2).[10]
Molecule design
The chemical structure of deucravacitinib contains a methyl amide in which all three hydrogen atoms are replaced by deuterium.[11]
Society and culture
Legal status
On 26 January 2023, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Sotyktu, intended for the treatment of moderate to severe psoriasis.[12] The applicant for this medicinal product is Bristol-Myers Squibb Pharma EEIG.[12] Deucravacitinib was approved for medical use in the European Union in March 2023.
References
- "Sotyktu". Therapeutic Goods Administration (TGA). 14 December 2022. Retrieved 15 April 2023.
- "Sotyktu (Bristol-Myers Squibb Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 13 January 2023. Retrieved 9 April 2023.
- "Notice: Multiple Additions to the Prescription Drug List (PDL) [2023-03-08]". Health Canada. 8 March 2023. Retrieved 21 March 2023.
- "Summary Basis of Decision - Sotyktu". Health Canada. 10 March 2023. Retrieved 24 April 2023.
- "Sotyktu- deucravacitinib tablet, film coated". DailyMed. 9 September 2022. Archived from the original on 28 September 2022. Retrieved 27 September 2022.
- "Sotyktu". Union Register of medicinal products. 27 March 2023. Retrieved 30 March 2023.
- "U.S. Food and Drug Administration Approves Sotyktu (deucravacitinib), Oral Treatment for Adults with Moderate-to-Severe Plaque Psoriasis" (Press release). Bristol Myers Squibb. 10 September 2022. Archived from the original on 10 September 2022. Retrieved 10 September 2022 – via Business Wire.
- "Drug Approval Package: Sotyktu". U.S. Food and Drug Administration (FDA). 14 October 2022. Retrieved 4 January 2023.
- "Advancing Health Through Innovation: New Drug Therapy Approvals 2022". U.S. Food and Drug Administration (FDA). 10 January 2023. Retrieved 22 January 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Chimalakonda A, Burke J, Cheng L, Catlett I, Tagen M, Zhao Q, et al. (October 2021). "Selectivity Profile of the Tyrosine Kinase 2 Inhibitor Deucravacitinib Compared with Janus Kinase 1/2/3 Inhibitors". Dermatology and Therapy. 11 (5): 1763–1776. doi:10.1007/s13555-021-00596-8. PMC 8484413. PMID 34471993.
- Mullard A (September 2022). "First de novo deuterated drug poised for approval". Nature Reviews. Drug Discovery. 21 (9): 623–625. doi:10.1038/d41573-022-00139-6. PMID 35974147. S2CID 251623586.
- "Sotyktu: Pending EC decision". European Medicines Agency (EMA). 26 January 2023. Retrieved 28 January 2023.