Polymerase
In biochemistry, a polymerase is an enzyme (EC 2.7.7.6/7/19/48/49) that synthesizes long chains of polymers or nucleic acids. DNA polymerase and RNA polymerase are used to assemble DNA and RNA molecules, respectively, by copying a DNA template strand using base-pairing interactions or RNA by half ladder replication.
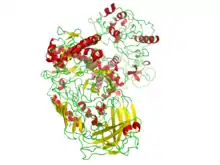
A DNA polymerase from the thermophilic bacterium, Thermus aquaticus (Taq) (PDB 1BGX, EC 2.7.7.7) is used in the polymerase chain reaction, an important technique of molecular biology.
A polymerase may be template-dependent or template-independent. Poly-A-polymerase is an example of template independent polymerase. Terminal deoxynucleotidyl transferase also known to have template independent and template dependent activities.
Types
By function
| DNA-polymerase | RNA-polymerase | |
|---|---|---|
| Template is DNA | DNA dependent DNA-polymerase or common DNA polymerases |
DNA dependent RNA-polymerase or common RNA polymerases |
| Template is RNA | RNA dependent DNA polymerase or Reverse transcriptase |
RNA dependent RNA polymerase or RdRp or RNA-replicase |
- DNA polymerase (DNA-directed DNA polymerase, DdDP)
- Family A: DNA polymerase I; Pol γ, θ, ν
- Family B: DNA polymerase II; Pol α, δ, ε, ζ
- Family C: DNA polymerase III holoenzyme
- Family X: Pol β, λ, μ
- Terminal deoxynucleotidyl transferase (TDT), which lends diversity to antibody heavy chains.[1]
- Family Y: DNA polymerase IV (DinB) and DNA polymerase V (UmuD'2C) - SOS repair polymerases; Pol η, ι, κ
- Reverse transcriptase (RT; RNA-directed DNA polymerase; RdDP)
- DNA-directed RNA polymerase (DdRP, RNAP)
- Multi-subunit (msDdRP): RNA polymerase I, RNA polymerase II, RNA polymerase III
- Single-subunit (ssDdRP): T7 RNA polymerase, POLRMT
- Primase, PrimPol
- RNA replicase (RNA-directed RNA polymerase, RdRP)
- Viral (single-subunit)
- Eukaryotic cellular (cRdRP; dual-subunit)
- Template-less RNA elongation
By structure
Polymerases are generally split into two superfamilies, the "right hand" fold (InterPro: IPR043502) and the "double psi beta barrel" (often simply "double-barrel") fold. The former is seen in almost all DNA polymerases and almost all viral single-subunit polymerases; they are marked by a conserved "palm" domain.[2] The latter is seen in all multi-subunit RNA polymerases, in cRdRP, and in "family D" DNA polymerases found in archaea.[3][4] The "X" family represented by DNA polymerase beta has only a vague "palm" shape, and is sometimes considered a different superfamily (InterPro: IPR043519).[5]
Primases generally don't fall into either category. Bacterial primases usually have the Toprim domain, and are related to topoisomerases and mitochondrial helicase twinkle.[6] Archae and eukaryotic primases form an unrelated AEP family, possibly related to the polymerase palm. Both families nevertheless associate to the same set of helicases.[7]
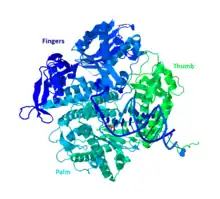 Right hand structure of Bacteriophage RB69, a family B DdRP.
Right hand structure of Bacteriophage RB69, a family B DdRP.
See also
References
- Loc'h J, Rosario S, Delarue M (September 2016). "Structural Basis for a New Templated Activity by Terminal Deoxynucleotidyl Transferase: Implications for V(D)J Recombination". Structure. 24 (9): 1452–63. doi:10.1016/j.str.2016.06.014. PMID 27499438.
- Hansen JL, Long AM, Schultz SC (August 1997). "Structure of the RNA-dependent RNA polymerase of poliovirus". Structure. 5 (8): 1109–22. doi:10.1016/S0969-2126(97)00261-X. PMID 9309225.
- Cramer P (February 2002). "Multisubunit RNA polymerases". Current Opinion in Structural Biology. 12 (1): 89–97. doi:10.1016/S0959-440X(02)00294-4. PMID 11839495.
- Sauguet L (September 2019). "The Extended "Two-Barrel" Polymerases Superfamily: Structure, Function and Evolution". Journal of Molecular Biology. 431 (20): 4167–4183. doi:10.1016/j.jmb.2019.05.017. PMID 31103775.
- Salgado PS, Koivunen MR, Makeyev EV, Bamford DH, Stuart DI, Grimes JM (December 2006). "The structure of an RNAi polymerase links RNA silencing and transcription". PLoS Biology. 4 (12): e434. doi:10.1371/journal.pbio.0040434. PMC 1750930. PMID 17147473.
- Aravind L, Leipe DD, Koonin EV (September 1998). "Toprim--a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins". Nucleic Acids Research. 26 (18): 4205–13. doi:10.1093/nar/26.18.4205. PMC 147817. PMID 9722641.
- Iyer LM, Koonin EV, Leipe DD, Aravind L (2005). "Origin and evolution of the archaeo-eukaryotic primase superfamily and related palm-domain proteins: structural insights and new members". Nucleic Acids Research. 33 (12): 3875–96. doi:10.1093/nar/gki702. PMC 1176014. PMID 16027112.