Anti-obesity medication
Anti-obesity medication or weight loss medications are pharmacological agents that reduce or control excess body fat. These medications alter one of the fundamental processes of the human body, weight regulation, by reducing appetite and consequently energy intake, increasing energy expenditure, redirecting nutrients from adipose to lean tissue, or interfering with the absorption of calories.[1][2][3] The main treatment modalities for overweight and individuals with obesity remain dieting (healthy diet and caloric restriction) and physical exercise.
| Part of a series on |
| Human body weight |
|---|
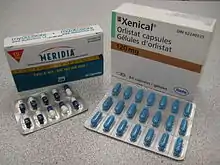
In the United States, orlistat (Xenical) and semaglutide (Wegovy) are approved by the FDA for long-term use.[4][5][6]
Because of potential side effects, and limited evidence of small benefits in weight reduction for children and adolescents with obesity,[7] it is recommended that anti-obesity medications only be prescribed for obesity where it is hoped that the benefits of the treatment outweigh its risks.[8][9] In the United States, the Food and Drug Administration advocates that people with either a body-mass index of at least 30, or a body-mass index of at least 27 with at least one weight-related comorbidity, represent a patient population with sufficiently high baseline health risks to justify the use of anti-obesity medication.[10]
Mechanisms of action
Energy intake
- Cannabinoid receptor antagonists were developed to treat obesity because researchers noticed that cannabinoid agonists (such as THC, the main pharmacologically active component of cannabis), increased appetite. However, some drugs in this class ceased development to concerns about mental health and suicide. More selective drugs—some are in development that act only in peripheral tissues, not the brain—may be able to achieve this result with fewer adverse effects.[11][12]
- Catecholamine releasing agents such as amphetamine, phentermine, and related substituted amphetamines (e.g., bupropion) act as appetite suppressants.[13][14]
- GLP-1 agonists such as tirzepatide, semaglutide, and liraglutide slow gastric emptying and also have neurologically driven effects on appetite.[15] It is unknown if GLP-1 agonists or dual/triple agonists of GLP-1 and/or the glucagon or GIP receptors act solely by reducing energy intake or if they also increase energy expenditure.[16]
- Setmelanotide is an agonist of the melanocortin 4 receptor and is used in people with certain rare genetic conditions that cause obesity. It is less effective and not approved for general obesity.[17]
Energy expenditure
- Adrenergic agonists, especially those that work on the beta-2 adrenergic receptor, increase energy expenditure. Although some such as clenbuterol are used without medical approval for weight loss, none have achieved approval for this indication due to cardiac risks.[18][19]
- The discontinued drug 2,4-dinitrophenol works by increasing energy expenditure by decreasing the efficiency of mitochondria (uncoupling agent).[18]
- Thyroid hormones, another early weight loss drug, also raised energy expenditure but ceased to be used for weight loss due to cardiac risks and other adverse effects.[18] Selective thyromimetics that work on the thyroid hormone receptor beta may be able to exert some of the beneficial thermogenic effects of thyroid hormones with fewer adverse effects, but none have received approval as of 2023.[16]
Others
- Methionine aminopeptidase 2 inhibitors cause weight loss in humans, which may be due to either energy intake, expenditure, or a combination of both. However, development was discontinued due to deaths in clinical trials.[18]
- Bimagrumab, an experimental drug, works by inhibiting the action of myostatin, which limits the size of skeletal muscle. The drug has shown the ability to increase lean mass simultaneously to decreasing fat mass in obese humans, which is beneficial because it preserves or increases energy expenditure while reducing risks associated with excess fat.[18]
History
The first described attempts at producing weight loss are those of Soranus of Ephesus, a Greek physician, in the second century AD. He prescribed elixirs of laxatives and purgatives, as well as heat, massage, and exercise. This remained the mainstay of treatment for well over a thousand years. It was not until the 1920s and 1930s that new treatments began to appear. Based on its effectiveness for hypothyroidism, thyroid hormone became a popular treatment for obesity in euthyroid people. It had a modest effect but produced the symptoms of hyperthyroidism as a side effect, such as palpitations and difficulty sleeping. 2,4-Dinitrophenol (DNP) was introduced in 1933; this worked by uncoupling the biological process of oxidative phosphorylation in mitochondria, causing them to produce heat instead of ATP. The most significant side effect was a sensation of warmth, frequently with sweating. Overdose, although rare, led to a rise in body temperature and, ultimately, fatal hyperthermia. By the end of 1938 DNP had fallen out of use because the FDA had become empowered to put pressure on manufacturers, who voluntarily withdrew it from the market.[20]
Amphetamines (marketed as Benzedrine) became popular for weight loss during the late 1930s. They worked primarily by suppressing appetite, and had other beneficial effects such as increased alertness. Use of amphetamines increased over the subsequent decades, including Obetrol and culminating in the "rainbow diet pill" regime.[21] This was a combination of multiple pills, all thought to help with weight loss, taken throughout the day. Typical regimens included stimulants, such as amphetamines, as well as thyroid hormone, diuretics, digitalis, laxatives, and often a barbiturate to suppress the side effects of the stimulants.[21] In 1967/1968 a number of deaths attributed to diet pills triggered a Senate investigation and the gradual implementation of greater restrictions on the market.[22] While rainbow diet pills were banned in the US in the late 1960s, they reappeared in South America and Europe in the 1980s.[21] Eventually rainbow diet pills were re-introduced into the US by the 2000s and led to additional adverse health effects.[23][24][25]
In 1959, phentermine had been FDA approved and fenfluramine in 1973. The two were no more popular than other medications until in 1992 a researcher reported that when combined the two caused a 10% weight loss which was maintained for more than two years.[26] Fen-phen was born and rapidly became the most commonly prescribed diet medication. Dexfenfluramine (Redux) was developed in the mid-1990s as an alternative to fenfluramine with fewer side-effects, and received regulatory approval in 1996. However, this coincided with mounting evidence that the combination could cause valvular heart disease in up to 30% of those who had taken it, leading to withdrawal of Fen-phen and dexfenfluramine from the market in September 1997.[22] Medical complications included fatal pulmonary hypertension and heart valve damage due to Redux and Fen-phen, and hemorrhagic stroke due to phenylpropanolamine.[27][28]
Since the introduction of medicines for the management of obesity in the 1930s, many compounds have been tried. Most of them reduce body weight by small amounts, and several of them are no longer marketed for obesity because of their side effects. Out of 25 anti-obesity medications withdrawn from the market between 1964 and 2009, 23 acted by altering the functions of chemical neurotransmitters in the brain. The most common side effects of these drugs that led to withdrawals were mental disturbances, cardiac side effects, and drug abuse or drug dependence. Deaths were reportedly associated with seven products.[29] Ephedra was removed from the US market in 2004 over concerns that it raises blood pressure and could lead to strokes and death.[30]
Medication
FDA approved
The FDA approves anti-obesity medications as an adjunctive therapy to diet and exercise for patients for whom lifestyle changes do not result in sufficient weight loss. In the United States, semaglutide (Wegovy) is approved by the FDA for chronic weight management.[31] The FDA guidelines say that a therapy may be approved if it results in weight loss that is statistically significant greater than placebo and generally at least 5 percent of body weight over six months that comes predominantly from fat mass.[18][32] Some other prescription weight loss medications are stimulants, which are recommended only for short-term use, and thus are of limited usefulness for patients who may need to reduce weight over months or years.[33] As of 2022, there is no pathway for approval for drugs that reduce fat mass without 5 percent overall weight loss, even if they significantly improve metabolic health; neither is there one for drugs that help patients maintain weight loss although this can be more challenging than losing weight.[18]
| Medication Name | Trade name(s) | Mechanism of action | Current FDA Status | placebo-adjusted percent bodyweight lost (highest dose studied) |
|---|---|---|---|---|
| Semaglutide | Wegovy | GLP-1 agonist | Approved for weight management (chronic) | 12%[34] |
| Phentermine/Topirimate | Qsymia | Phentermine is a substituted amphetamine and topiramate has an unknown mechanism of action | Approved for weight management (short-term) | 10%[35] |
| Naltrexone/bupropion | Contrave | Approved for weight management (chronic) | 5%[36] | |
| Liraglutide | Saxenda | GLP-1 agonist | Approved for weight management (chronic) | 7 kilograms (15 lb) (percentage bodyweight not provided)[37] |
| Gelesis100 | Plenity | Oral hydrogel | Approved for weight management (chronic) | 2%[38] |
| Orlistat | Xenical | Absorption inhibitor | Approved for weight management (chronic) | 3 kilograms (6.6 lb); percentage not provided[39] |
| Phentermine | Substituted amphetamine | Approved for weight management (short-term) | [data missing] | |
| Methamphetamine | Desoxyn | Substituted amphetamine | Approved for weight management (short-term) |
Not FDA approved for weight loss
| Medication Name | Trade name(s) | Mechanism of action | Current FDA Status | placebo-adjusted percent bodyweight lost (highest dose studied) |
|---|---|---|---|---|
| Retatrutide | GLP-1, GIP, and glucagon receptor triple agonist | In clinical trials | 24 percent in a Phase II trial[40] | |
| Exenatide | Byetta | GLP-1 agonist | Approved for type 2 diabetes | [data missing] |
| Cetilistat | Absorption inhibitor | Not approved | - | |
| Lorcaserin | Belviq | 5-HT2C receptor agonist | Withdrawn for safety reasons | - |
| Sibutramine | Meridia | Serotonin–norepinephrine reuptake inhibitor | Withdrawn for safety reasons | - |
| Rimonabant | Acomplia, Zimbutli | Cannabinoid receptor antagonist | Withdrawn for safety reasons | - |
| Fenfluramine | Serotonin releasing agent | Withdrawn for safety reasons | - | |
| Fenfluramine/phentermine (fen-phen) | Pondimin | Withdrawn for safety reasons | ||
| Dexfenfluramine | Redux | Serotonin releasing agent | Withdrawn for safety reasons | |
| Metformin | Glucophage | Unknown | Approved for type 2 diabetes | [data missing] |
| Pramlintide | Amylin analogue | Approved for type 2 diabetes | [data missing] | |
| Tirzepatide | Mounjaro | GLP-1 and GIP double agonist | Approved for type 2 diabetes | 18%[41][42] |
| Thyroid hormones | Thyroid hormone receptor agonist | Approved for people with hypothyroidism, not for weight loss in people with normal thyroid function | [43] | |
| 2,4-Dinitrophenol | Uncoupling agent | Never approved, for safety reasons | 17.1 pounds (7.8 kg) per patient on average[44][45] | |
| Amphetamine salts | Obetrol | Approved 1960, withdrawn 1973; Adderall was later approved for ADHD and narcolepsy and is still used for those purposes | ||
| Digoxin (a digitalis leaf extract) | Lanoxin | Approved for certain cardiac indications and termination of fetal heartbeat; appetite reduction is a side effect | [46] | |
| Phenylpropanolamine | Was an over-the-counter medication ingredient | Withdrawn in 2005 due to risk of hemorragic stroke | ||
| Ephedrine | Adrenergic agonist | Approved for asthma[47] | ||
| ECA stack | Combination of ephedrine and caffeine, sometimes adding aspirin | Around 4–6 kilograms (8.8–13.2 lb)[48] | ||
| Ephedra | Plant extract sold as a dietary supplement | Contains ephedrine, an adrenergic agonist | Banned in 2004 for safety reasons | 0.9 kilograms (2.0 lb) per month more than placebo[48] |
GLP-1 analogs and agonists
Glucagon-like peptide-1 (GLP-1) are peptide incretin hormones involved in blood sugar control.[49]
In 2021, one review concluded that "Currently, gut peptide analogues such as semaglutide [...] and [...] tirzepatide are the furthest advanced in clinical development".[50] In 2022, a further review found that these two peptides are "the most promising candidates for the upcoming battle in the anti-obesity market".[51] An article in The New York Times notes the high costs for semaglutide and potentially tirzepatide, suggesting that many people "who could most benefit from weight loss may be unable to afford such expensive drugs".[52]
Semaglutide
Semaglutide (Ozempic) is a GLP-1 analogue, administered once weekly. It is more effective than exenatide.[53] In June 2021, the US Food and Drug Administration (FDA) approved semaglutide injection sold under the brand name Wegovy for long-term weight management in adults.[54][55] Wegovy was approved for medical use ("used together with diet and physical activity") in the European Union in January 2022.[56]
Tirzepatide
A phase III clinical trial indicated that tirzepatide could be used for substantial weight loss.[51] Specifically, phase-3 clinical trials found that after 71 weeks patients had lost 16% of their starting body weight on average.[34] On 13 May 2022, it was approved under the name Mounjaro for type-2 diabetes (though not specifically for weight loss) .[57]
Exenatide
Exenatide (Byetta) is a long-acting analogue of the hormone GLP-1, which the intestines secrete in response to the presence of food. Among other effects, GLP-1 delays stomach emptying and promotes a feeling of fullness after eating. Some people with obesity are deficient in GLP-1, and dieting reduces GLP-1 further.[58] Byetta is currently available as a treatment for Diabetes mellitus type 2. Some, but not all, patients find that they lose substantial weight when taking Byetta. Drawbacks of Byetta include that it must be injected subcutaneously twice daily, and that it causes severe nausea in some patients, especially when therapy is initiated. As of 2015, Byetta was recommended only for patients with Type 2 Diabetes.[59]
Liraglutide
Liraglutide (Saxenda) is another GLP-1 analogue for daily administration, approved 2014.
Orforglipron
Orforglipron is a non-peptide GLP-1 agonist of potential interest because it can be taken orally and is chemically simpler and thus potentially cheaper than peptides such as semaglutide.
Orlistat
Orlistat (Xenical) reduces intestinal fat absorption by inhibiting the enzyme pancreatic lipase. Frequent oily bowel movements steatorrhea is a possible side effect of using Orlistat. But if fat in the diet is reduced, symptoms often improve. Originally available only by prescription, it was approved by the FDA for over-the-counter sale in February 2007.[60] On 26 May 2010, the U.S. Food and Drug Administration (FDA) has approved a revised label for Xenical to include new safety information about cases of severe liver injury that have been reported rarely with the use of this medication.[61] Of the 40 million users of orlistat worldwide, 13 cases of severe liver damage have been reported.[62]
Cetilistat
Cetilistat is a medication designed to treat obesity. It acts in the same way as the older medication orlistat by inhibiting pancreatic lipase, an enzyme that breaks down triglycerides in the intestine. Without this enzyme, triglycerides from the diet are prevented from being hydrolyzed into absorbable free fatty acids and are excreted undigested.[63]
A 2010 phase 2 trial found cetilistat significantly reduced weight and was better tolerated than orlistat.[64] It has not been approved in the US.
Phentermine/topiramate
The combination of phentermine and topiramate, brand name Qsymia (formerly Qnexa) was approved by the U.S. FDA on 17 July 2012, as an obesity treatment complementary to a diet and exercise regimen.[65] In October 2012, the European Medicines Agency, by contrast, rejected the combination (Qsiva) as a treatment for obesity, citing concerns about long-term effects on the heart and blood vessels, mental health and cognitive side-effects.[66]
Naltrexone/bupropion
Naltrexone/bupropion is a combination medication used for weight loss in those that have either obesity or overweight with some weight-related illnesses. It combines low doses of bupropion and naltrexone. Both medications have individually shown some evidence of effectiveness in weight loss, and the combination has been shown to have some synergistic effects on weight. In September 2014, a sustained release formulation of the medication was approved for marketing in the United States under the brand name Contrave.[67] The combination was approved for use in the European Union in March 2015, under the brand name Mysimba.[68]
Gelesis100
Gelesis100 (sold under the brand name "Plenity") is an oral superabsorbent hydrogel used for weight loss in the treatment of obesity and overweight.[69] As Gelesis100 absorbs water, it expands in the stomach and small bowel, which may result in satiety. Gelesis100 was approved in April 2019 by the US Food and Drug Administration as a medical device. In 2022, the American Gastroenterology Association recommended the use of Gelesis100 be limited to clinical trials due to limited evidence.[70]
Lorcaserin
Lorcaserin (Belviq) was approved 28 June 2012 for obesity with other co-morbidities. The average weight loss by study participants was modest, but the most common side effects of the medication are considered benign.[71] It reduces appetite by activating a type of serotonin receptor known as the 5-HT2C receptor in a region of the brain called the hypothalamus, which is known to control appetite.[72] This drug has now been withdrawn from the market because a safety clinical trial shows an increased occurrence of cancer.[73]
Sibutramine
Sibutramine (Meridia), which acts in the brain to inhibit deactivation of the neurotransmitters, thereby decreasing appetite was approved in 1997 and withdrawn from the United States and Canadian markets in October 2010 due to cardiovascular concerns.[5][74]
As late as 2004, some held that Meridia was a harmless medication for fighting obesity: the US District Court of the Northern District of Ohio rejected 113 cases complaining about the negative effects of the medication, stating that the clients lacked supporting facts and that the representatives involved were not qualified enough.[75]
In October Sibutramine was withdrawn from the market for cardiovascular side effects like stroke or heart attack, sometimes fatal, in the United States,[76] the UK,[77] the EU,[78] Australia,[79] Canada,[80] Hong Kong[81] and Colombia.[82]
Rimonabant
Rimonabant (also known as SR141716; trade names Acomplia and Zimulti)[83] was an anorectic antiobesity medication that was first approved in Europe in 2006 but was withdrawn worldwide in 2008 due to serious psychiatric side effects; it was never approved in the United States.[84][85] It works via a specific blockade of the endocannabinoid system. It has been developed from the knowledge that cannabis smokers often experience hunger, which is often referred to as "the munchies".[86]
Rimonabant is an inverse agonist for the cannabinoid receptor CB1 and was the first medication approved in that class.[87][88]
Off label and experimental medications
- Metformin
- In people with type 2 diabetes mellitus and for those taking clozapine for schizophrenia, the medication metformin (Glucophage) can reduce weight, but in others it is not approved as an anti-obesity medication.[89][90] Metformin limits the amount of glucose that is produced by the liver as well as increases muscle consumption of glucose. It also helps in increasing the body's response to insulin.[91]
- Tesofensine (NS2330)
- A serotonin–noradrenaline–dopamine reuptake inhibitor from the phenyltropane family of medications, which as of 2009, was being developed for the treatment of obesity.[92] Tesofensine was originally developed by a Danish biotechnology company, NeuroSearch, who transferred the rights to Saniona in 2014.[93] Tesofensine has been evaluated in Phase 1 and Phase 2 human clinical studies with the aim of investigating treatment potential with regards to obesity.
- Amylin analog pramlinatide
- Pramlintide, originally developed by Amylin Pharmaceuticals, now owned by AstraZeneca Pharmaceuticals, is an injectable analogue of amylin (secreted by the Beta cells of the pancreas in a fixed ratio when insulin is released and activated) an approved for treating diabetes type 1 and 2. It is in testing for treating obesity in non-diabetics.
Nutraceuticals, herbal and alternative medicine
Dietary supplements, foodstuffs, or programs for weight loss are heavily promoted through advertisements in print, on television, and on the internet. The US Food and Drug Administration recommends caution with use of these products,[94] since many of the claims of safety and effectiveness are unsubstantiated, and many of the studies purporting to demonstrate their effectiveness are funded by the manufactures and suffer a high degree of bias.[95] Individuals with anorexia nervosa or bulimia nervosa, and some athletes, try to control body weight with diet pills, laxatives, or diuretic medications, although the latter two generally have no impact on body fat and only cause short-lived weight-loss through dehydration.[96] Both diuretics and laxatives can cause electrolyte abnormalities which may cause cognitive, heart, and muscle problems, and can be fatal.
Canadian clinical practice 2006 guidelines state that there is insufficient evidence to recommend in favor of or against using herbal medicine, dietary supplements or homeopathy against obesity.[97] Some botanical supplements include high dosages of compounds found in plants with stimulant effects including yohimbine and higenamine.[98][99]
Quality control
Many products marketed as botanical weight loss supplements actually contain unapproved stimulants including analogues of amphetamine,[100] methamphetamine[101] and ephedra.[102]
Caffeine, coffee and green tea
Caffeine, coffee and green tea can suppress appetite (the hormone ghrelin) and decrease caloric intake (food consumption).[103][104][105][106] It can also cause beneficial changes in fat metabolism or lipolytic actions.[105][106][107] According to a review, habitual intake of 3 to 4 cups of coffee appears to be safe and to be associated with the most robust beneficial effects.[108] Thermogenic actions and, in the case of the caffeinated drink mate (yerba maté), prolonged gastric emptying time may also play a role.[107]
Berberin
Berberine can be useful in obesity treatment and prevention through a range of mechanisms, including effects on PEPCK, the gut microbiome, AMPK and glucose metabolism.[109] A review found that berberine can elicit clinical benefits for various diseases at standard doses and has low toxicity. The (possibly relatively small but useful) anti-obesity effects (as well as effects on associationed issues such as via effects on insulin receptors) are thought to partly stem from its effects on the microbiome, such as enrichment of butyrate-producing bacteria which can upregulate GLP-1 and PYY.[110] Berberine can modulate the diversity of the gut microbiome at the dose of 500 mg/day.[109]
Forskolin
Forskolin increases cAMP levels. Results from clinical trials "lead to speculation that forskolin might be helpful in the management of overweight". While it apparently reduced body fat levels in people with obesity, additional trials with high quality are required.[111][112]
Oleuropein
Oleuropein, as contained in olive leaf extract, has anti-obesity properties, which may make it useful as a supportive treatment.[113][114]
Caloric restriction mimetic
Accumulating evidence demonstrated anti-obesity impact of caloric restriction mimetics (CRMs) like spermidine. Such nutraceuticals exert effects similar to caloric restriction.[115] However, more studies are needed and some CRMs may not have effects useful for the treatment or prevention of obesity – for resveratrol in specific "current evidence does not fully support its use to prevent or treat age- or obesity-related diseases".[116]
Conjugated linoleic acid
Conjugated linoleic acid is claimed to help reduce obesity but it is ineffective for this use.[117]
ECA stack
The ECA Stack cannot be marketed in most developed countries but used to be marketed as a weight loss aid; it provided modest short term weight loss but evidence for the long term was lacking. Additionally there was a risk of adverse effects on the cardiovascular, mental, digestive, and nervous systems.[118]
Side effects
Some anti-obesity medications can have severe, even, lethal side effects, fen-phen being a famous example. Fen-phen was reported through the FDA to cause abnormal echocardiograms, heart valve problems, and rare valvular diseases.[120] One of, if not the first, to sound alarms was Sir Arthur MacNalty, Chief Medical Officer (United Kingdom). As early as the 1930s, he warned against the use of 2,4-Dinitrophenol as an anti-obesity medication and the injudicious and/or medically unsupervised use of thyroid hormone to achieve weight reduction.[121][122] The side effects are often associated with the medication's mechanism of action. In general, stimulants carry a risk of high blood pressure, faster heart rate, palpitations, closed-angle glaucoma, drug addiction, restlessness, agitation, and insomnia.
Another medication, orlistat, blocks absorption of dietary fats, and as a result may cause oily spotting bowel movements (steatorrhea), oily stools, stomach pain, and flatulence.[123] A similar medication designed for patients with Type 2 diabetes is Acarbose; which partially blocks absorption of carbohydrates in the small intestine, and produces similar side effects including stomach pain and flatulence.[124]
See also
References
- Ryan, Donna H. (30 September 2021). "Next Generation Antiobesity Medications: Setmelanotide, Semaglutide, Tirzepatide and Bimagrumab: What do They Mean for Clinical Practice?". Journal of Obesity & Metabolic Syndrome. 30 (3): 196–208. doi:10.7570/jomes21033. ISSN 2508-6235.
- Jimenez-Munoz, Carlos M.; López, Marta; Albericio, Fernando; Makowski, Kamil (6 May 2021). "Targeting Energy Expenditure—Drugs for Obesity Treatment". Pharmaceuticals. 14 (5): 435. doi:10.3390/ph14050435. ISSN 1424-8247.
- National Institute for Health and Clinical Excellence. Clinical guideline 43: Obesity: The prevention, identification, assessment and management of overweight and obesity in adults and children. London, 2006.
- "WIN – Publication – Prescription Medications for the Treatment of Obesity". National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). National Institutes of Health. Archived from the original on 13 January 2009. Retrieved 14 January 2009.
- "www.fda.gov". Food and Drug Administration. Archived from the original on 18 January 2017. Retrieved 16 December 2019.
- "FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014". FDA. 21 June 2021. Archived from the original on 4 June 2021. Retrieved 19 July 2022.
- Mead E, Atkinson G, Richter B, Metzendorf MI, Baur L, Finer N, et al. (November 2016). "Drug interventions for the treatment of obesity in children and adolescents". The Cochrane Database of Systematic Reviews. 11 (11): CD012436. doi:10.1002/14651858.CD012436. PMC 6472619. PMID 27899001.
- Snow V, Barry P, Fitterman N, Qaseem A, Weiss K (April 2005). "Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians". Annals of Internal Medicine. 142 (7): 525–531. doi:10.7326/0003-4819-142-7-200504050-00011. PMID 15809464.
- Cooke D, Bloom S (November 2006). "The obesity pipeline: current strategies in the development of anti-obesity drugs". Nature Reviews. Drug Discovery. 5 (11): 919–931. doi:10.1038/nrd2136. PMID 17080028. S2CID 28733429.
- Colman E (February 2007). "Guidance for Industry Developing Products for Weight Management". Food and Drug Administration. Archived from the original on 13 October 2022. Retrieved 19 July 2022.
- Rohbeck, Elisabeth; Eckel, Juergen; Romacho, Tania (1 March 2021). [10.1152/physiol.00029.2020 "Cannabinoid Receptors in Metabolic Regulation and Diabetes"]. Physiology. 36 (2): 102–113. doi:10.1152/physiol.00029.2020. ISSN 1548-9213.
{{cite journal}}: Check|url=value (help) - Nguyen, Thuy; Thomas, Brian F.; Zhang, Yanan (2019). "Overcoming the psychiatric side effects of the cannabinoid CB1 receptor antagonists: current approaches for therapeutics development". Current topics in medicinal chemistry. 19 (16): 1418–1435. doi:10.2174/1568026619666190708164841. ISSN 1568-0266.
- "Evekeo Prescribing Information" (PDF). Arbor Pharmaceuticals LLC. April 2014. pp. 1–2. Archived (PDF) from the original on 4 March 2016. Retrieved 11 August 2015.
- Bray GA (October 1993). "Use and abuse of appetite-suppressant drugs in the treatment of obesity". Annals of Internal Medicine. 119 (7 Pt 2): 707–713. doi:10.7326/0003-4819-119-7_Part_2-199310011-00016. PMID 8363202. S2CID 39529451.
- Shah M, Vella A (September 2014). "Effects of GLP-1 on appetite and weight". Reviews in Endocrine & Metabolic Disorders. 15 (3): 181–187. doi:10.1007/s11154-014-9289-5. PMC 4119845. PMID 24811133.
- Genchi, V. A.; Palma, G.; Sorice, G. P.; D’Oria, R.; Caccioppoli, C.; Marrano, N.; Biondi, G.; Caruso, I.; Cignarelli, A.; Natalicchio, A.; Laviola, L.; Giorgino, F.; Perrini, S. (1 November 2023). "Pharmacological modulation of adaptive thermogenesis: new clues for obesity management?". Journal of Endocrinological Investigation. 46 (11): 2213–2236. doi:10.1007/s40618-023-02125-0. ISSN 1720-8386.
- Son, Jang Won; Kim, Sungrae (23 December 2020). "Comprehensive Review of Current and Upcoming Anti-Obesity Drugs". Diabetes & Metabolism Journal. 44 (6): 802–818. doi:10.4093/dmj.2020.0258. Retrieved 15 October 2023.
- Christoffersen, Berit Østergaard; Sanchez‐Delgado, Guillermo; John, Linu Mary; Ryan, Donna H.; Raun, Kirsten; Ravussin, Eric (April 2022). "Beyond appetite regulation: Targeting energy expenditure, fat oxidation, and lean mass preservation for sustainable weight loss". Obesity. 30 (4): 841–857. doi:10.1002/oby.23374. ISSN 1930-7381.
- Kumari, Sweta; Pal, Biplab; Sahu, Sanjeev Kumar; Prabhakar, Pranav Kumar; Tewari, Devesh (1 July 2023). "Adverse events of clenbuterol among athletes: a systematic review of case reports and case series". International Journal of Legal Medicine. 137 (4): 1023–1037. doi:10.1007/s00414-023-02996-1. ISSN 1437-1596.
- Parascandola J (November 1974). "Dinitrophenol and bioenergetics: an historical perspective". Molecular and Cellular Biochemistry. 5 (1–2): 69–77. doi:10.1007/BF01874175. PMID 4610359. S2CID 2656970.
- Cohen PA, Goday A, Swann JP (September 2012). "The return of rainbow diet pills". American Journal of Public Health. 102 (9): 1676–1686. doi:10.2105/AJPH.2012.300655. PMC 3482033. PMID 22813089.
- Pool, Robert (2001). Fat: Fighting the Obesity Epidemic. Oxford, UK: Oxford University Press. ISBN 978-0-19-511853-7.
- Cohen PA (March 2009). "Imported fenproporex-based diet pills from Brazil: a report of two cases". Journal of General Internal Medicine. 24 (3): 430–433. doi:10.1007/s11606-008-0878-4. PMC 2642570. PMID 19096898.
- Cohen PA, McCormick D, Casey C, Dawson GF, Hacker KA (June 2009). "Imported compounded diet pill use among Brazilian women immigrants in the United States". Journal of Immigrant and Minority Health. 11 (3): 229–236. doi:10.1007/s10903-007-9099-x. PMID 18066718. S2CID 8730835.
- Smith BR, Cohen PA (May 2010). "Dependence on the Brazilian diet pill: a case report". The American Journal on Addictions. 19 (3): 291–292. doi:10.1111/j.1521-0391.2010.00034.x. PMID 20525038. S2CID 32173764.
- Weintraub M (May 1992). "Long-term weight control: the National Heart, Lung, and Blood Institute funded multimodal intervention study". Clinical Pharmacology and Therapeutics. 51 (5): 581–585. doi:10.1038/clpt.1992.68. PMID 1445528. S2CID 221606104.
- Abenhaim L, Moride Y, Brenot F, Rich S, Benichou J, Kurz X, et al. (August 1996). "Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International Primary Pulmonary Hypertension Study Group". The New England Journal of Medicine. 335 (9): 609–616. doi:10.1056/NEJM199608293350901. PMID 8692238.
- Fishman AP (1999). "Aminorex to fen/phen: an epidemic foretold". Circulation. 99 (1): 156–161. doi:10.1161/01.CIR.99.1.156. PMID 9884392.
- Onakpoya, Igho J.; Heneghan, Carl J.; Aronson, Jeffrey K. (2016). "Post-marketing withdrawal of anti-obesity medicinal products because of adverse drug reactions: a systematic review". BMC Medicine. 14 (1): 191. doi:10.1186/s12916-016-0735-y. ISSN 1741-7015. PMC 5126837. PMID 27894343.
- Kolata, Gina (2007). Rethinking thin: The new science of weight loss – and the myths and realities of dieting. Picador. ISBN 978-0-312-42785-6.
- Office of the Commissioner (21 June 2021). "FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014". FDA. Archived from the original on 4 June 2021. Retrieved 19 July 2022.
- Haslam, D. (March 2016). "Weight management in obesity – past and present". International Journal of Clinical Practice. 70 (3): 206–217. doi:10.1111/ijcp.12771. ISSN 1368-5031.
- "Obesity Medication: Gastrointestinal Agents, Other, CNS Stimulants, Anorexiants, Glucagon-like Peptide-1 Agonists, Antidepressants, dopamine reuptake inhibitors; opioid antagonists". emedicine.medscape.com. Archived from the original on 4 November 2016. Retrieved 2 November 2016.
- Wilding JP, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. (March 2021). "Once-Weekly Semaglutide in Adults with Overweight or Obesity". The New England Journal of Medicine. 384 (11): 989–1002. doi:10.1056/NEJMoa2032183. PMID 33567185. S2CID 231883214. Archived from the original on 18 March 2023. Retrieved 21 February 2023.
- Smith SM, Meyer M, Trinkley KE (March 2013). "Phentermine/topiramate for the treatment of obesity". The Annals of Pharmacotherapy. 47 (3): 340–349. doi:10.1345/aph.1R501. PMID 23482732. S2CID 30461611.
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. (COR-I Study Group) (August 2010). "Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial". Lancet. 376 (9741): 595–605. doi:10.1016/S0140-6736(10)60888-4. PMID 20673995. S2CID 28588741.
- Astrup A, Rössner S, Van Gaal L, Rissanen A, Niskanen L, Al Hakim M, et al. (NN8022-1807 Study Group) (November 2009). "Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study". Lancet. 374 (9701): 1606–1616. doi:10.1016/S0140-6736(09)61375-1. PMID 19853906. S2CID 12897343.
- Greenway FL, Aronne LJ, Raben A, Astrup A, Apovian CM, Hill JO, et al. (February 2019). "A Randomized, Double-Blind, Placebo-Controlled Study of Gelesis100: A Novel Nonsystemic Oral Hydrogel for Weight Loss". Obesity. 27 (2): 205–216. doi:10.1002/oby.22347. PMC 6587502. PMID 30421844.
- Padwal RS, Majumdar SR (January 2007). "Drug treatments for obesity: orlistat, sibutramine, and rimonabant". Lancet. 369 (9555): 71–77. doi:10.1016/S0140-6736(07)60033-6. PMID 17208644. S2CID 35104831.
- Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, et al. (June 2023). "Triple-Hormone-Receptor Agonist Retatrutide for Obesity - A Phase 2 Trial". The New England Journal of Medicine. doi:10.1056/NEJMoa2301972. PMID 37366315. Free access subject to registration.
- Frías JP, Davies MJ, Rosenstock J, Pérez Manghi FC, Fernández Landó L, Bergman BK, et al. (August 2021). "Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes". The New England Journal of Medicine. 385 (6): 503–515. doi:10.1056/NEJMoa2107519. PMID 34170647. S2CID 235635529.
- Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. (July 2022). "Tirzepatide Once Weekly for the Treatment of Obesity". The New England Journal of Medicine. 387 (3): 205–216. doi:10.1056/NEJMoa2206038. PMID 35658024. S2CID 249385412.
- "Is it Safe to Take Thyroid Hormones for Weight Loss?". Archived from the original on 19 February 2023. Retrieved 19 February 2023.
- Tainter, M. L. (3 August 1935). "DINITROPHENOL IN THE TREATMENT OF OBESITY: FINAL REPORT". Journal of the American Medical Association. 105 (5): 332. doi:10.1001/jama.1935.02760310006002.
- "FDA targets unlawful internet sales of illegal prescription medicines during International Operation Pangea IX". Food and Drug Administration. 24 March 2020. Archived from the original on 19 February 2023. Retrieved 19 February 2023.
- David MNV; Shetty, M. (2023). "Digoxin". PMID 32310485. Archived from the original on 27 August 2022. Retrieved 19 February 2023.
- "Role of OTC Asthma Medications in the Community Pharmacy". Pharmacy Times. 8 December 2021. Retrieved 24 October 2023.
- Eckerson, Joan M. (2015). "Weight Loss Nutritional Supplements". Nutritional Supplements in Sports and Exercise. Springer International Publishing. pp. 159–185. ISBN 978-3-319-18230-8.
- "FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes". U.S. Food and Drug Administration (FDA) (Press release). 13 May 2022. Archived from the original on 13 May 2022. Retrieved 13 May 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Finer N (November 2021). "Future directions in obesity pharmacotherapy". European Journal of Internal Medicine. 93: 13–20. doi:10.1016/j.ejim.2021.04.024. PMID 34024701. S2CID 235167933.
- Jung HN, Jung CH (March 2022). "The Upcoming Weekly Tides (Semaglutide vs. Tirzepatide) against Obesity: STEP or SURPASS?". Journal of Obesity & Metabolic Syndrome. 31 (1): 28–36. doi:10.7570/jomes22012. PMC 8987449. PMID 35314521.
- Kolata G (28 April 2022). "Patients Taking Experimental Obesity Drug Lost More Than 50 Pounds, Maker Claims". The New York Times. Archived from the original on 27 June 2022. Retrieved 13 May 2022.
- Ahmann, Andrew J.; Capehorn, Matthew; Charpentier, Guillaume; Dotta, Francesco; Henkel, Elena; Lingvay, Ildiko; Holst, Anders G.; Annett, Miriam P.; Aroda, Vanita R. (1 February 2018). "Efficacy and safety of once-Weekly semaglutide versus exenatide ER in subjects with type 2 diabetes (SUSTAIN 3): A 56-Week, open-Label, randomized clinical trial". Diabetes Care. 41 (2): 258–266. doi:10.2337/dc17-0417. ISSN 1935-5548. PMID 29246950. S2CID 1010366.
- "Wegovy- semaglutide injection, solution". DailyMed. 4 June 2021. Archived from the original on 14 December 2021. Retrieved 11 March 2022.
- "FDA Approves New Drug Treatment for Chronic Weight Management, First Since 2014". U.S. Food and Drug Administration (FDA) (Press release). 4 June 2021. Archived from the original on 4 June 2021. Retrieved 5 June 2021.
- "Wegovy EPAR". European Medicines Agency (EMA). 11 November 2021. Archived from the original on 2 July 2022. Retrieved 11 March 2022.
- Office of the Commissioner (13 May 2022). "FDA Approves Novel, Dual-Targeted Treatment for Type 2 Diabetes". FDA. Archived from the original on 13 May 2022. Retrieved 19 July 2022.
- de Luis DA, Gonzalez Sagrado M, Conde R, Aller R, Izaola O (2007). "Decreased basal levels of glucagon-like peptide-1 after weight loss in obese subjects". Annals of Nutrition & Metabolism. 51 (2): 134–138. doi:10.1159/000103273. PMID 17536190. S2CID 25707685.
- Sauer N, Reining F, Schulze Zur Wiesch C, Burkhardt T, Aberle J (July 2015). "Off-label antiobesity treatment in patients without diabetes with GLP-1 agonists in clinical practice". Hormone and Metabolic Research. 47 (8): 560–564. doi:10.1055/s-0034-1387793. PMID 25230325. S2CID 20174195.
- "Press Announcements". Fda.gov. 8 (10): 1845. 1970. Bibcode:1970JPoSB...8.1845.. doi:10.1002/pol.1970.160081020. Archived from the original on 13 May 2009. Retrieved 16 December 2019.
- "FDA Drug Safety Communication: Completed safety review of Xenical/Alli (orlistat) and severe liver injury". fda.gov. Archived from the original on 24 April 2019. Retrieved 16 December 2019.
- Aronne LJ, Powell AG, Apovian CM (September 2011). "Emerging pharmacotherapy for obesity". Expert Opinion on Emerging Drugs. 16 (3): 587–596. doi:10.1517/14728214.2011.609168. PMID 21834735. S2CID 9253331.
- Yamada Y, Kato T, Ogino H, Ashina S, Kato K (August 2008). "Cetilistat (ATL-962), a novel pancreatic lipase inhibitor, ameliorates body weight gain and improves lipid profiles in rats". Hormone and Metabolic Research. 40 (8): 539–543. doi:10.1055/s-2008-1076699. PMID 18500680. S2CID 29076657.
- Kopelman P, Groot G, Rissanen A, Rossner S, Toubro S, Palmer R, et al. (January 2010). "Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical)". Obesity. 18 (1): 108–115. doi:10.1038/oby.2009.155. PMID 19461584. S2CID 205526626.
- Salynn Boyles (17 July 2012). "FDA approves diet drug Qsymia : agency warns of increased risk for oral birth defects". WebMD. Archived from the original on 20 February 2020. Retrieved 14 November 2012., citing "FDA approves weight-management drug Qsymia" (Press release). U.S. Food and Drug Administration. 17 July 2012. Archived from the original on 18 January 2017. Retrieved 16 December 2019.
- "Qsiva". EMA. 13 June 2013. Archived from the original on 12 December 2022. Retrieved 12 December 2022.
- "Contrave Extended-Release- naltrexone hydrochloride and bupropion hydrochloride tablet, extended release". DailyMed. 26 April 2019. Archived from the original on 4 June 2020. Retrieved 5 August 2020.
- "Mysimba EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 22 October 2020. Retrieved 5 August 2020.
- Pass A, Bialonczyk D, Chiquette E, Goldman JD (September 2021). "Oral Superabsorbent Hydrogel (Plenity) for Weight Management". The Annals of Pharmacotherapy. 55 (9): 1146–1152. doi:10.1177/1060028020983046. PMID 33348994. S2CID 229351539.
- Grunvald, E; Shah, R; Hernaez, R; Chandar, AK; Pickett-Blakely, O; Teigen, LM; Harindhanavudhi, T; Sultan, S; Singh, S; Davitkov, P; AGA Clinical Guidelines, Committee (November 2022). "AGA Clinical Practice Guideline on Pharmacological Interventions for Adults With Obesity". Gastroenterology. 163 (5): 1198–1225. doi:10.1053/j.gastro.2022.08.045. PMID 36273831. S2CID 253052479.
- Bray GA, Frühbeck G, Ryan DH, Wilding JP (May 2016). "Management of obesity". Lancet. 387 (10031): 1947–1956. doi:10.1016/S0140-6736(16)00271-3. PMID 26868660. S2CID 21805769.
- Shukla AP, Kumar RB, Aronne LJ (2015). "Lorcaserin Hcl for the treatment of obesity". Expert Opinion on Pharmacotherapy. 16 (16): 2531–2538. doi:10.1517/14656566.2015.1096345. PMID 26472579. S2CID 44520532.
- "FDA requests the withdrawal of the weight-loss drug Belviq, Belviq XR (lorcaserin) from the market". www.fda.gov. 19 February 2019. Archived from the original on 20 December 2020. Retrieved 23 December 2020.
- "Recalls and safety alerts". Health Canada. Government of Canada. 23 October 2012. Archived from the original on 23 October 2020. Retrieved 31 August 2020.
- Obesity, Fitness & Wellness Week (14 August 2004). "Legal Issues; Court dismisses claims against anti-obesity medication". Biotech Week: 11. ISSN 1535-2757.
- Rockoff JD, Dooren JC (8 October 2010). "Abbott Pulls Diet Drug Meridia Off US Shelves". The Wall Street Journal. Archived from the original on 11 October 2010. Retrieved 8 October 2010.
- "Top obesity drug sibutramine being suspended". BBC News. 22 January 2010. Archived from the original on 25 January 2010. Retrieved 22 January 2010.
- "Sibutramin-Vertrieb in der Europäischen Union ausgesetzt" [Sibutramine sales suspended in the European Union]. Abbott Laboratories (in German). Germany. 21 January 2010. Archived from the original on 19 July 2012. Retrieved 27 January 2010.
- "Sibutramine (brand name Reductil) Information – Australia". Abbott Laboratories. 2010. Archived from the original on 14 October 2010. Retrieved 8 October 2010.
- "MedEffect Canada - Advisories, Warnings and Recalls - Health Canada". hc-sc.gc.ca. 9 December 2002. Archived from the original on 11 January 2013. Retrieved 19 November 2010.
- "De-registration of pharmaceutical products containing sibutramine" (Press release). info.gov in Hong Kong. 2 November 2010. Archived from the original on 21 October 2011. Retrieved 8 November 2010.
- "Top 10 Diet Pills That Work in 2014". TENMANIA. 2014. Archived from the original on 20 April 2014. Retrieved 15 April 2014.
- "Rimonabant". AdisInsight. Archived from the original on 22 February 2017. Retrieved 21 February 2017.
- Sam AH, Salem V, Ghatei MA (2011). "Rimonabant: From RIO to Ban". Journal of Obesity. 2011: 432607. doi:10.1155/2011/432607. PMC 3136184. PMID 21773005.
- Moreira FA, Crippa JA (June 2009). "The psychiatric side-effects of rimonabant". Revista Brasileira de Psiquiatria. 31 (2): 145–153. doi:10.1590/s1516-44462009000200012. PMID 19578688.
- Wadman M (January 2006). "Rimonabant adds appetizing choice to slim obesity market". Nature Medicine. 12 (1): 27. doi:10.1038/nm0106-27. PMID 16397550. S2CID 5298220.
- Fong TM, Heymsfield SB (September 2009). "Cannabinoid-1 receptor inverse agonists: current understanding of mechanism of action and unanswered questions". International Journal of Obesity. 33 (9): 947–955. doi:10.1038/ijo.2009.132. PMID 19597516.
- "European Approval Comes Early for Sanofi-Aventis' Acomplia". IHS. 23 June 2006. Archived from the original on 22 February 2017. Retrieved 21 February 2017.
- Bray GA, Greenway FL (December 1999). "Current and potential drugs for treatment of obesity". Endocrine Reviews. 20 (6): 805–875. doi:10.1210/edrv.20.6.0383. PMID 10605627.
- Siskind, Dan J.; Leung, Janni; Russell, Anthony W.; Wysoczanski, Daniel; Kisely, Steve (15 June 2016). Holscher, Christian (ed.). "Metformin for clozapine associated obesity: a systematic review and meta-analysis". PLOS ONE. 11 (6): e0156208. Bibcode:2016PLoSO..1156208S. doi:10.1371/journal.pone.0156208. ISSN 1932-6203. PMC 4909277. PMID 27304831.
- "Diabetes Medications: Metformin" (PDF). Archived from the original (PDF) on 8 July 2015. Retrieved 21 October 2015.
- Doggrell SA (July 2009). "Tesofensine--a novel potent weight loss medicine. Evaluation of: Astrup A, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1906-13" (PDF). Expert Opinion on Investigational Drugs. 18 (7): 1043–1046. doi:10.1517/13543780902967632. PMID 19548858. S2CID 207475155. Archived (PDF) from the original on 5 April 2020. Retrieved 29 November 2019.
- "NeuroSearch A/S signs agreement to transfer Phase I-II projects NS2359 and NS2330 (Tesofensine)". newsclient.omxgroup.com. Archived from the original on 27 April 2016. Retrieved 24 April 2017.
- "The Facts About Weight Loss Products and Programs". U.S. Food and Drug Administration. Archived from the original on 11 August 2007.
- "Prepared Statement of the Federal Trade Commission on the Marketing of Dietary Supplements" (Press release). Committee on Governmental Affairs, United States Senate. 8 October 2002. Archived from the original on 25 August 2006. Retrieved 7 August 2006.
- Martin M, Schlabach G, Shibinski K (January 1998). "The Use of Nonprescription Weight Loss Products Among Female Basketball, Softball, and Volleyball Athletes from NCAA Division I Institutions: Issues and Concerns". Journal of Athletic Training. 33 (1): 41–44. PMC 1320374. PMID 16558483.
- Lau DC, Douketis JD, Morrison KM, Hramiak IM, Sharma AM, Ur E (April 2007). "2006 Canadian clinical practice guidelines on the management and prevention of obesity in adults and children [summary]". CMAJ. 176 (8): S1-13. doi:10.1503/cmaj.061409. PMC 1839777. PMID 17420481.
- Cohen PA, Wang YH, Maller G, DeSouza R, Khan IA (March 2016). "Pharmaceutical quantities of yohimbine found in dietary supplements in the USA". Drug Testing and Analysis. 8 (3–4): 357–369. doi:10.1002/dta.1849. PMID 26391406.
- Cohen PA, Travis JC, Keizers PH, Boyer FE, Venhuis BJ (February 2019). "The stimulant higenamine in weight loss and sports supplements". Clinical Toxicology. 57 (2): 125–130. doi:10.1080/15563650.2018.1497171. PMID 30188222. S2CID 52165506.
- Cohen PA, Bloszies C, Yee C, Gerona R (March 2016). "An amphetamine isomer whose efficacy and safety in humans has never been studied, β-methylphenylethylamine (BMPEA), is found in multiple dietary supplements". Drug Testing and Analysis. 8 (3–4): 328–333. doi:10.1002/dta.1793. PMID 25847603. S2CID 205762285.
- Cohen PA, Travis JC, Venhuis BJ (July 2014). "A methamphetamine analog (N,α-diethyl-phenylethylamine) identified in a mainstream dietary supplement". Drug Testing and Analysis. 6 (7–8): 805–807. doi:10.1002/dta.1578. PMID 24124092. S2CID 42232885.
- Cohen PA, Avula B, Venhuis B, Travis JC, Wang YH, Khan IA (January 2017). "Pharmaceutical doses of the banned stimulant oxilofrine found in dietary supplements sold in the USA". Drug Testing and Analysis. 9 (1): 135–142. doi:10.1002/dta.1976. PMID 27062112. S2CID 9463413.
- Schubert MM, Irwin C, Seay RF, Clarke HE, Allegro D, Desbrow B (December 2017). "Caffeine, coffee, and appetite control: a review". International Journal of Food Sciences and Nutrition. 68 (8): 901–912. doi:10.1080/09637486.2017.1320537. hdl:10072/345209. PMID 28446037. S2CID 12944242.
- Harpaz E, Tamir S, Weinstein A, Weinstein Y (January 2017). "The effect of caffeine on energy balance". Journal of Basic and Clinical Physiology and Pharmacology. 28 (1): 1–10. doi:10.1515/jbcpp-2016-0090. PMID 27824614. S2CID 23963486.
- Sirotkin AV, Kolesárová A (April 2021). "The anti-obesity and health-promoting effects of tea and coffee". Physiological Research. 70 (2): 161–168. doi:10.33549/physiolres.934674. PMC 8820582. PMID 33992045.
- Dinh TC, Thi Phuong TN, Minh LB, Minh Thuc VT, Bac ND, Van Tien N, et al. (March 2019). "The effects of green tea on lipid metabolism and its potential applications for obesity and related metabolic disorders - An existing update". Diabetes & Metabolic Syndrome. 13 (2): 1667–1673. doi:10.1016/j.dsx.2019.03.021. PMID 31336539. S2CID 87224638.
- Tucci, Sonia A. (March 2010). "Phytochemicals in the Control of Human Appetite and Body Weight". Pharmaceuticals. 3 (3): 748–763. doi:10.3390/ph3030748. ISSN 1424-8247. PMC 4033978. PMID 27713277.
- O'Keefe JH, DiNicolantonio JJ, Lavie CJ (May 2018). "Coffee for Cardioprotection and Longevity" (PDF). Progress in Cardiovascular Diseases. 61 (1): 38–42. doi:10.1016/j.pcad.2018.02.002. PMID 29474816.
- Ilyas Z, Perna S, Al-Thawadi S, Alalwan TA, Riva A, Petrangolini G, et al. (July 2020). "The effect of Berberine on weight loss in order to prevent obesity: A systematic review". Biomedicine & Pharmacotherapy. 127: 110137. doi:10.1016/j.biopha.2020.110137. PMID 32353823. S2CID 218468722.
- Zhang L, Wu X, Yang R, Chen F, Liao Y, Zhu Z, et al. (2021). "Effects of Berberine on the Gastrointestinal Microbiota". Frontiers in Cellular and Infection Microbiology. 10: 588517. doi:10.3389/fcimb.2020.588517. PMC 7933196. PMID 33680978.
- Kamohara S (1 July 2016). "An evidence-based review: Anti-obesity effects of Coleus forskohlii". Personalized Medicine Universe. 5: 16–20. doi:10.1016/j.pmu.2016.02.001. ISSN 2186-4950.
- Ríos-Hoyo A, Gutiérrez-Salmeán G (June 2016). "New Dietary Supplements for Obesity: What We Currently Know". Current Obesity Reports. 5 (2): 262–270. doi:10.1007/s13679-016-0214-y. PMID 27053066. S2CID 12071766.
- Nani A, Murtaza B, Sayed Khan A, Khan NA, Hichami A (February 2021). "Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms". Molecules. 26 (4): 985. doi:10.3390/molecules26040985. PMC 7918790. PMID 33673390.
- Vogel P, Kasper Machado I, Garavaglia J, Zani VT, de Souza D, Morelo Dal Bosco S (December 2014). "Polyphenols benefits of olive leaf (Olea europaea L) to human health". Nutricion Hospitalaria. 31 (3): 1427–1433. doi:10.3305/nh.2015.31.3.8400. PMID 25726243.
- Voglhuber J, Ljubojevic-Holzer S, Abdellatif M, Sedej S (2021). "Targeting Cardiovascular Risk Factors Through Dietary Adaptations and Caloric Restriction Mimetics". Frontiers in Nutrition. 8: 758058. doi:10.3389/fnut.2021.758058. PMC 8514725. PMID 34660673.
- Pallauf K, Günther I, Kühn G, Chin D, de Pascual-Teresa S, Rimbach G (June 2021). "The Potential of Resveratrol to Act as a Caloric Restriction Mimetic Appears to Be Limited: Insights from Studies in Mice". Advances in Nutrition. 12 (3): 995–1005. doi:10.1093/advances/nmaa148. PMC 8166566. PMID 33271594.
- Onakpoya IJ, Posadzki PP, Watson LK, Davies LA, Ernst E (March 2012). "The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials". European Journal of Nutrition (Systematic review). 51 (2): 127–134. doi:10.1007/s00394-011-0253-9. PMID 21990002. S2CID 39625058.
- Shekelle PG, Hardy ML, Morton SC, Maglione M, Mojica WA, Suttorp MJ, et al. (March 2003). "Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance: a meta-analysis". JAMA. 289 (12): 1537–1545. doi:10.1001/jama.289.12.1470. PMID 12672771.
- Bray GA, Greenway FL (December 1999). "Current and potential drugs for treatment of obesity". Endocrine Reviews. 20 (6): 805–875. doi:10.1210/edrv.20.6.0383. PMID 10605627. Archived from the original on 5 September 2007. Retrieved 24 August 2007.
- Bachorik L. "FDA Announces Withdrawal Fenfluramine and Dexfenfluramine (Fen-Phen)". U.S. Food and Drug Administration. FDA. Archived from the original on 4 November 2009. Retrieved 27 January 2014.
- "Dangers of Slimming: A Medical Warning to Women". Examiner. Launceston, Tasmania, Australia. 21 January 1938. p. 14. Archived from the original on 7 October 2021.
However, the sex which for many years injured its health by tight lacing is not likely to be deterred from slimming by such considerations, The dictates of fashion will be paramount Sir Arthur was particularly concerned with the neurological side effects of the then popular practice of dosing with thyroid extract to lose weight and, also, use of the then much vaunted weight loss medication dinitrophenol, which his report found killed as many patients as it reduced in girth, as well as, the compromise of the malnourished person's immune system and their consequent, often, inability to resist infectious diseases like the then endemic tuberculosis (archaic "epidemics of consumption"
- "Death Reduces as Slimming Passes: Warning of Dangers, British Vital Statistics". Sidney Morning Herald. 17 November 1937. p. 10. Archived from the original on 4 March 2020.
- Johnson K. "Alli: A Weight Loss Drug". WebMD. Archived from the original on 2 February 2014. Retrieved 27 January 2014.
- "acarbose - oral, Precose". MedicineNet. Archived from the original on 1 February 2014. Retrieved 27 January 2014.
Further reading
Boss, Olivier; Karl G. Hofbauer (2004). Pharmacotherapy of obesity: options and alternatives. Boca Raton: CRC Press. ISBN 978-0-415-30321-7.
External links
 Media related to Anti-obesity medication at Wikimedia Commons
Media related to Anti-obesity medication at Wikimedia Commons- Prescription Medications for the Treatment of Obesity