Ammonium polyphosphate
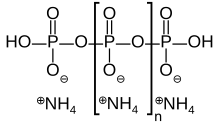
Ammonium polyphosphate is an inorganic salt of polyphosphoric acid and ammonia containing both chains and possibly branching. Its chemical formula is H(NH4PO3)nOH showing that each monomer consists of an orthophosphate radical of a phosphorus atom with three oxygens and one negative charge neutralized by an ammonium cation leaving two bonds free to polymerize. In the branched cases some monomers are missing the ammonium anion and instead link to three other monomers.
 | |
| Names | |
|---|---|
| Other names
Exolit AP 422, FR CROS 484, CS FR APP 231 | |
| Identifiers | |
| ChEBI | |
| ECHA InfoCard | 100.063.425 |
| E number | E452(v) (thickeners, ...) |
CompTox Dashboard (EPA) |
|
| Properties | |
| [NH4PO3]n(OH)2 | |
| Molar mass | 97.01 g/mol |
| Appearance | white powder |
| Density | 1,9 g/cm3; bulk density = 0,7 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
The properties of ammonium polyphosphate depend on the number of monomers in each molecule and to a degree on how often it branches. Shorter chains (n < 100) are more water sensitive and less thermally stable than longer chains (n > 1000),[1] but short polymer chains (e.g. pyro-, tripoly-, and tetrapoly-) are more soluble and show increasing solubility with increasing chain length.[2]
Ammonium polyphosphate can be prepared by reacting concentrated phosphoric acid with ammonia. However, iron and aluminum impurities, soluble in concentrated phosphoric acid, form gelatinous precipitates or "sludges" in ammonium polyphosphate at pH between 5 and 7.[3] Other metal impurities such as copper, chromium, magnesium, and zinc form granular precipitates.[4] However, depending on the degree of polymerization, ammonium polyphosphate can act as a chelating agent to keep certain metal ions dissolved in solution.[5]
Ammonium polyphosphate is used as a food additive, emulsifier, (E number: E545) and as a fertilizer.
Ammonium polyphosphate (APP) is also used as a flame retardant in many applications such as paints and coatings, and in a variety of polymers: the most important ones are polyolefins, and particularly polypropylene, where APP is part of intumescent systems.[6] Compounding with APP-based flame retardants in polypropylene is described in.[7] Further applications are thermosets, where APP is used in unsaturated polyesters and gel coats (APP blends with synergists), epoxies and polyurethane castings (intumescent systems). APP is also applied to flame retard polyurethane foams.
Ammonium polyphosphates used as flame retardants in polymers have long chains and a specific crystallinity (Form II). They start to decompose at 240 °C to form ammonia and phosphoric acid. The phosphoric acid acts as an acid catalyst in the dehydration of carbon-based poly-alcohols, such as cellulose in wood. The phosphoric acid reacts with alcohol groups to form heat-unstable phosphate esters. The esters decompose to release carbon dioxide and regenerate the phosphoric acid catalyst . In the gas phase, the release of non-flammable carbon dioxide helps to dilute the oxygen of the air and flammable decomposition products of the material that is burning. In the condensed phase, the resultant carbonaceous char helps to shield the underlying polymer from attack by oxygen and radiant heat.[8] Use as an intumescent is achieved when combined with starch-based materials such as pentaerythritol and melamine as expanding agents. The mechanisms of intumescence and the mode of action of APP are described in a series of publications.[9][10]
References
- Archived 2010-05-22 at the Wayback Machine
- US 4041133, Young, Donald C., "Ammonium polyphosphate production", published 1977-08-09, assigned to Union Oil Co. of California
- US 4721519, Thomas, William P. & Lawton, William S., "Stable ammonium polyphosphate liquid fertilizer from merchant grade phosphoric acid", published 1988-01-26, assigned to American Petro Mart Inc.
- US 3044851, Young, Donald C., "Production of ammonium phosphates and product thereof", published 1962-07-17, assigned to Collier Carbon & Chemical Co.
- Gowariker, Vasant; Krishnamurthy, V. N.; Gowariker, Sudha; Dhanorkar, Manik; Paranjape, Kalyani (8 April 2009). The Fertilizer Encyclopedia. John Wiley & Sons. ISBN 9780470431764. Retrieved 30 June 2018 – via Google Books.
- Weil, E.D., Levchik, S.V. Flame retardants for plastics and textiles, p. 16. Hanser Publishers, Munich, Germany, 2009
- "As a flame retardant". Mindfully.org. Archived from the original on 13 September 2007. Retrieved 30 June 2018.
- US 4515632, Maurer, Alexander & Staendeke, Horst, "Activated ammonium polyphosphate, a process for making it, and its use", published 1985-05-07, assigned to Hoechst AG
- Camino, G.; Luda, M.P. Mechanistic study of intumescence, p. 48 f, in Le Bras, M.; Camino, G.; Bourbigot, S.; Delobel, R. Eds., Fire retardancy of polymers; The use of intumescence, The Royal Society of Chemistry, Cambridge, UK, 1998
- Bourbigot, S.; Le Bras, M. Intumescence flame retardants and char formation, p. 139 f, in Troitzsch, J. Ed. Plastics flammability handbook, 3rd Ed., Hanser Publishers, Munich, 2004
External links
- US 2950961, Striplin Jr., Marcus M.; Stinson, John M. & Potts, John M., "Production of liquid fertilizers", published 1960-08-30, assigned to Tennessee Valley Authority
- US 4211546, Jensen, William C., "Process for preparation of ammonium polyphosphate", published 1980-07-08, assigned to Western Farm Services Inc.
- Specialchem4polymers.com