Firazorexton
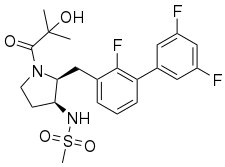
Firazorexton (INNTooltip International Nonproprietary Name; development code TAK-994) is an experimental orexin 2 (OX2) receptor agonist first described in a 2019 patent filed by Takeda Pharmaceutical Company.[1][2]
 | |
| Clinical data | |
|---|---|
| Other names | TAK-994 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C22H25F3N2O4S |
| Molar mass | 470.51 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Firazorexton was studied by Takeda for the treatment of narcolepsy.[3][4][5] It is a small-molecule and orally active compound and acts as a highly selective agonist of the orexin receptor 2 (OX2) with >700-fold selectivity over the orexin receptor 1 (OX1).[3][4][6][7] Firazorexton is related to danavorexton (TAK-925).[8] The compound reached phase 2 clinical trials for narcolepsy.[9] However, clinical development was discontinued in October 2021 for safety reasons.[8][10][11][12][13]
References
- "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO Drug Information. 34 (1): 93–269. 2020.
Proposed INN: List 123
- WO application 2019027058, Kajita Y, Mikami S, Miyanohana Y, Koike T, Daini M, Oyabu N, Ogino M, Takeuchi K, Ito Y, Tokunaga N, Sugimoto T, Miyazaki T, Oda T, Hoashi Y, Hattori Y, Imamura K, "Heterocyclic compound and use therof", published 2019-02-07, assigned to Takeda Pharmaceutical Company
- Ishikawa T, Suzuki M, Kimura H (April 2020). "0141 A Novel, Orally Available Orexin 2 Receptor-Selective Agonist, TAK-994, Shows Wake-Promoting Effects Following Chronic Dosing in an Orexin-Deficient Narcolepsy Mouse Model". Sleep. 43 (Supplement 1): A56. doi:10.1093/sleep/zsaa056.139. eISSN 1550-9109. ISSN 0161-8105.
- Thorpy MJ (January 2020). "Recently Approved and Upcoming Treatments for Narcolepsy". CNS Drugs. 34 (1): 9–27. doi:10.1007/s40263-019-00689-1. PMC 6982634. PMID 31953791.
- Ishikawa, Takashi; Hara, Hiroe; Kawano, Ayumi; Tohyama, Kimio; Kajita, Yuichi; Miyanohana, Yuhei; Koike, Tatsuki; Kimura, Haruhide (2023). "TAK-994, a Novel Orally Available Brain-Penetrant Orexin 2 Receptor-Selective Agonist, Suppresses Fragmentation of Wakefulness and Cataplexy-Like Episodes in Mouse Models of Narcolepsy". Journal of Pharmacology and Experimental Therapeutics. 385 (3): 193–204. doi:10.1124/jpet.122.001449. PMID 37001988. S2CID 257879743.
- Zhang D, Perrey DA, Decker AM, Langston TL, Mavanji V, Harris DL, et al. (June 2021). "Discovery of Arylsulfonamides as Dual Orexin Receptor Agonists". Journal of Medicinal Chemistry. 64 (12): 8806–8825. doi:10.1021/acs.jmedchem.1c00841. PMC 8994207. PMID 34101446.
- Pellitteri G, de Biase S, Valente M, Gigli GL (August 2021). "How treatable is narcolepsy with current pharmacotherapy and what does the future hold?". Expert Opinion on Pharmacotherapy. 22 (12): 1517–1520. doi:10.1080/14656566.2021.1915987. PMID 33882765. S2CID 233349300.
- Jacobson LH, Hoyer D, de Lecea L (January 2022). "Hypocretins (orexins): The ultimate translational neuropeptides". J Intern Med. 291 (5): 533–556. doi:10.1111/joim.13406. PMID 35043499. S2CID 248119793.
- Clinical trial number NCT04096560 for "A Study of TAK-994 in Adults With Type 1 and Type 2 Narcolepsy" at ClinicalTrials.gov
- "Takeda Provides Update on TAK-994 Clinical Program". Business Wire. 5 October 2021.
- "Takeda pauses TAK-994 clinical studies due to safety glitch". The Pharma Letter. 6 October 2021.
- Jackson M (6 October 2021). "Takeda Halts Phase II Studies For Key R&D Asset TAK-994 In Narcolepsy". Scrip. Informa Pharma Intelligence.
- Tong A (6 October 2021). "Takeda flashes red light on 'breakthrough' narcolepsy drug after PhII trials turned up mysterious safety signal". Endpoints News.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.