Vornorexant
Vornorexant, also known by its developmental code names ORN-0829 and TS-142, is an orexin antagonist medication which is under development for the treatment of insomnia and sleep apnea.[3][4][5] It is a dual orexin OX1 and OX2 receptor antagonist (DORA).[5][6] The medication is taken by mouth.[1] As of June 2021, vornorexant is in phase 2 clinical trials for insomnia and phase 1 trials for sleep apnea.[3] It is under development by Taisho Pharmaceutical.[3]
 | |
| Clinical data | |
|---|---|
| Other names | ORN-0829; TS-142 |
| Routes of administration | By mouth[1] |
| Drug class | Orexin antagonist |
| Pharmacokinetic data | |
| Elimination half-life | 1.3–3.3 hours[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
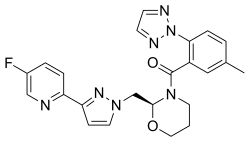
| Formula | C23H22FN7O2 |
| Molar mass | 447.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Vornorexant has a time to peak of 2.5 hours and a relatively short elimination half-life of 1.3 to 3.3 hours.[1][2] It was designed to have a short half-life and duration in order to reduce next-day side effects like somnolence.[5]
See also
- Seltorexant – another investigational short-acting orexin receptor antagonist
- List of investigational sleep drugs § Orexin receptor antagonists
References
- Uchiyama M, Kambe D, Imadera Y, Kajiyama Y, Ogo H, Uchimura N (March 2022). "Effects of TS-142, a novel dual orexin receptor antagonist, on sleep in patients with insomnia: a randomized, double-blind, placebo-controlled phase 2 study". Psychopharmacology (Berl). doi:10.1007/s00213-022-06089-6. PMID 35296912.
- Uchiyama, M; Kambe, D; Imadera, Y; Sunaga, H; Hasegawa, S; Nogi, T; Kajiyama, Y; Yoshida, S; Ogo, H; Uchimura, N (April 2020). "0146 Efficacy and Safety of Single Dose of TS-142, a Novel and Potent Dual Orexin Receptor Antagonist, in Insomnia Patients". Sleep. 43 (Supplement 1): A58–A58. doi:10.1093/sleep/zsaa056.144. eISSN 1550-9109. ISSN 0161-8105.
- "TS 142 - AdisInsight".
- Muehlan C, Vaillant C, Zenklusen I, Kraehenbuehl S, Dingemanse J (November 2020). "Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders". Expert Opin Drug Metab Toxicol. 16 (11): 1063–1078. doi:10.1080/17425255.2020.1817380. PMID 32901578. S2CID 221572078.
- Jacobson LH, Hoyer D, de Lecea L (January 2022). "Hypocretins (orexins): The ultimate translational neuropeptides". J Intern Med. doi:10.1111/joim.13406. PMID 35043499.
- Futamura A, Suzuki R, Tamura Y, Kawamoto H, Ohmichi M, Hino N, Tokumaru Y, Kirinuki S, Hiyoshi T, Aoki T, Kambe D, Nozawa D (July 2020). "Discovery of ORN0829, a potent dual orexin 1/2 receptor antagonist for the treatment of insomnia". Bioorg Med Chem. 28 (13): 115489. doi:10.1016/j.bmc.2020.115489. PMID 32482533. S2CID 216517776.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.