Heterotrimeric G protein
Heterotrimeric G protein, also sometimes referred to as the "large" G proteins (as opposed to the subclass of smaller, monomeric small GTPases) are membrane-associated G proteins that form a heterotrimeric complex. The biggest non-structural difference between heterotrimeric and monomeric G protein is that heterotrimeric proteins bind to their cell-surface receptors, called G protein-coupled receptors, directly. These G proteins are made up of alpha (α), beta (β) and gamma (γ) subunits.[1] The alpha subunit is attached to either a GTP or GDP, which serves as an on-off switch for the activation of G-protein.
| Heterotrimeric G-protein GTPase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.6.5.1 | ||||||||
| CAS no. | 9059-32-9 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
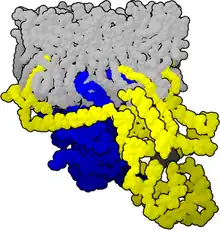
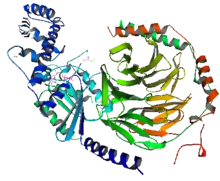
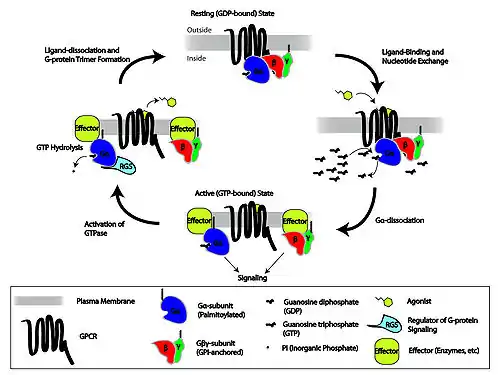
When ligands bind a GPCR, the GPCR acquires GEF (guanine nucleotide exchange factor) ability, which activates the G-protein by exchanging the GDP on the alpha subunit to GTP. The binding of GTP to the alpha subunit results in a structural change and its dissociation from the rest of the G-protein. Generally, the alpha subunit binds membrane-bound effector proteins for the downstream signaling cascade, but the beta-gamma complex can carry out this function also. G-proteins are involved in pathways such as the cAMP/PKA pathway, ion channels, MAPK, PI3K.
There are four main families of G proteins: Gi/Go, Gq, Gs, and G12/13.[2]
Alpha subunits
Reconstitution experiments carried out in the early 1980s showed that purified Gα subunits can directly activate effector enzymes. The GTP form of the α subunit of transducin (Gt) activates the cyclic GMP phosphodiesterase from retinal rod outer segments,[3] and the GTP form of the α subunit of the stimulatory G protein (Gs) activates hormone-sensitive adenylate cyclase.[4][5] More than one type of G protein co-exist in the same tissue. For example, in adipose tissues, two different G-proteins with interchangeable beta-gamma complexes are used to activate or inhibit adenylyl cyclase. The alpha subunit of a stimulatory G protein activated by receptors for stimulatory hormones could stimulate adenylyl cyclase, which activates cAMP used for downstream signal cascades. While on the other hand, the alpha subunit of an inhibitory G protein activated by receptors of inhibitory hormones could inhibit adenylyl cyclase, which blocks downstream signal cascades.
Gα subunits consist of two domains, the GTPase domain, and the alpha-helical domain.
There exist at least 20 different Gα subunits, which are separated into four main groups. This nomenclature is based on their sequence homologies:[6]
| G-protein family | α-subunit | Gene | Signal transduction | Use/Receptors (examples) | Effects (examples) |
|---|---|---|---|---|---|
| Gi-family (InterPro: IPR001408) | |||||
| Gi/o | αi, αo | GNAO1, GNAI1, GNAI2, GNAI3 | Inhibition of adenylate cyclase, opens K+-channels (via β/γ subunits), closes Ca2+-channels | Muscarinic M2 and M4,[7] chemokine receptors, α2-Adrenoreceptors, Serotonin 5-HT1 receptors, Histamine H3 and H4, Dopamine D2-like receptors, type 2 cannabinoid receptors (CB2)[8] | Smooth muscle contraction, depress neuronal activity, interleukin secretion by human leukocytes[8] |
| Gt | αt (Transducin) | GNAT1, GNAT2 | Activation of phosphodiesterase 6 | Rhodopsin | Vision |
| Ggust | αgust (Gustducin) | GNAT3 | Activation of phosphodiesterase 6 | Taste receptors | Taste |
| Gz | αz | GNAZ | Inhibition of adenylate cyclase | Platelets | Maintaining the ionic balance of perilymphatic and endolymphatic cochlear fluids. |
| Gs-family (InterPro: IPR000367) | |||||
| Gs | αs | GNAS | Activation of adenylate cyclase | Beta-adrenoreceptors; Serotonin 5-HT4, 5-HT6 and 5-HT7; Dopamine D1-like receptors, Histamine H2, Vasopressin V2, type 2 cannabinoid receptors [8] | Increase heart rate, Smooth muscle relaxation, stimulate neuronal activity, interleukin secretion by human leukocytes [8] |
| Golf | αolf | GNAL | Activation of adenylate cyclase | olfactory receptors | Smell |
| Gq-family (InterPro: IPR000654) | |||||
| Gq | αq, α11, α14, α15, α16 | GNAQ, GNA11, GNA14, GNA15 | Activation of phospholipase C | α1-Adrenoreceptors, Muscarinic M1, M3, and M5,[7] Histamine H1, Serotonin 5-HT2 , Vasopressin V1 receptors | Smooth muscle contraction, Ca2+ flux |
| G12/13-family (InterPro: IPR000469) | |||||
| G12/13 | α12, α13 | GNA12, GNA13 | Activation of the Rho family of GTPases | Cytoskeletal functions, Smooth muscle contraction | |
G beta-gamma complex
The β and γ subunits are closely bound to one another and are referred to as the G beta-gamma complex. Both beta and gamma subunits have different isoforms, and some combination of isoforms result in dimerization while other combinations do not. For example, beta1 binds both gamma subunits while beta3 binds neither.[9] Upon activation of the GPCR, the Gβγ complex is released from the Gα subunit after its GDP-GTP exchange.
Function
The free Gβγ complex can act as a signaling molecule itself, by activating other second messengers or by gating ion channels directly.
For example, the Gβγ complex, when bound to histamine receptors, can activate phospholipase A2. Gβγ complexes bound to muscarinic acetylcholine receptors, on the other hand, directly open G protein-coupled inward rectifying potassium channels (GIRKs).[10] When acetylcholine is the extracellular ligand in the pathway, the heart cell hyperpolarizes normally to decrease heart muscle contraction. When substances such as muscarine act as ligands, the dangerous amount of hyperpolarization leads to hallucination. Therefore, proper functioning of Gβγ plays a key role in our physiological well-being. The last function is activating L-type calcium channels, as in H3 receptor pharmacology.
Heterotrimeric G-proteins in plants
Heterotrimeric G-protein signaling in plants deviates from the metazoan model at various levels. For example, the presence of extra-Large G alpha, loss of G alpha and Regulator of G-protein signaling (RGS) in many plant lineages.[11] In addition, the G-proteins are not essential for the survival in dicotyledonous plants, while they are essential for the survival of monocotyledonous plants.
References
- Hurowitz EH, Melnyk JM, Chen YJ, Kouros-Mehr H, Simon MI, Shizuya H (April 2000). "Genomic characterization of the human heterotrimeric G protein alpha, beta, and gamma subunit genes". DNA Research. 7 (2): 111–20. doi:10.1093/dnares/7.2.111. PMID 10819326.
- Nature Reviews Drug Discovery GPCR Questionnaire Participants (July 2004). "The state of GPCR research in 2004". Nature Reviews. Drug Discovery (3 ed.). 3 (7): 575, 577–626. doi:10.1038/nrd1458. PMID 15272499. S2CID 33620092.
- Fung BK, Hurley JB, Stryer L (January 1981). "Flow of information in the light-triggered cyclic nucleotide cascade of vision". Proceedings of the National Academy of Sciences of the United States of America. 78 (1): 152–6. Bibcode:1981PNAS...78..152F. doi:10.1073/pnas.78.1.152. PMC 319009. PMID 6264430.
- Cerione RA, Sibley DR, Codina J, Benovic JL, Winslow J, Neer EJ, Birnbaumer L, Caron MG, Lefkowitz RJ, et al. (August 1984). "Reconstitution of a hormone-sensitive adenylate cyclase system. The pure beta-adrenergic receptor and guanine nucleotide regulatory protein confer hormone responsiveness on the resolved catalytic unit". The Journal of Biological Chemistry. 259 (16): 9979–82. doi:10.1016/S0021-9258(18)90913-0. PMID 6088509.
- May DC, Ross EM, Gilman AG, Smigel MD (December 1985). "Reconstitution of catecholamine-stimulated adenylate cyclase activity using three purified proteins". The Journal of Biological Chemistry. 260 (29): 15829–33. doi:10.1016/S0021-9258(17)36333-0. PMID 2999139.
- Strathmann MP, Simon MI (July 1991). "G alpha 12 and G alpha 13 subunits define a fourth class of G protein alpha subunits". Proceedings of the National Academy of Sciences of the United States of America. 88 (13): 5582–6. Bibcode:1991PNAS...88.5582S. doi:10.1073/pnas.88.13.5582. PMC 51921. PMID 1905812.
- Qin K, Dong C, Wu G, Lambert NA (August 2011). "Inactive-state preassembly of G(q)-coupled receptors and G(q) heterotrimers". Nature Chemical Biology. 7 (10): 740–7. doi:10.1038/nchembio.642. PMC 3177959. PMID 21873996.
- Saroz, Yurii; Kho, Dan T.; Glass, Michelle; Graham, Euan Scott; Grimsey, Natasha Lillia (2019-10-19). "Cannabinoid Receptor 2 (CB 2 ) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes". ACS Pharmacology & Translational Science. 2 (6): 414–428. doi:10.1021/acsptsci.9b00049. ISSN 2575-9108. PMC 7088898. PMID 32259074.
- Schmidt CJ, Thomas TC, Levine MA, Neer EJ (July 1992). "Specificity of G protein beta and gamma subunit interactions". The Journal of Biological Chemistry. 267 (20): 13807–10. doi:10.1016/S0021-9258(19)49638-5. PMID 1629181.
- Gulati S, Jin H, Masuho I, Orban T, Cai Y, Pardon E, Martemyanov KA, Kiser PD, Stewart PL, Ford CP, Steyaert J, Palczewski K (2018). "Targeting G protein-coupled receptor signaling at the G protein level with a selective nanobody inhibitor". Nature Communications. 9 (1): 1996. Bibcode:2018NatCo...9.1996G. doi:10.1038/s41467-018-04432-0. PMC 5959942. PMID 29777099.
- Mohanasundaram, Boominathan; Dodds, Audrey; Kukshal, Vandna; Jez, Joseph M; Pandey, Sona (4 April 2022). "Distribution and the evolutionary history of G-protein components in plant and algal lineages". Plant Physiology: kiac153. doi:10.1093/plphys/kiac153. PMC 9237705. PMID 35377452.
External links
- Heterotrimeric+G-Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- EC 3.6.5.1