Isoleucine
Isoleucine (symbol Ile or I)[1] is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a hydrocarbon side chain with a branch (a central carbon atom bound to three other carbon atoms). It is classified as a non-polar, uncharged (at physiological pH), branched-chain, aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it. Essential amino acids are necessary in our diet. In plants isoleucine can be synthesized from threonine and methionine.[2] In plants and bacteria, isoleucine is synthesized from pyruvate employing leucine biosynthesis enzymes.[3] It is encoded by the codons AUU, AUC, and AUA.
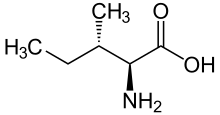 skeletal formula of L-isoleucine | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Isoleucine | |||
| Other names
(2S,3S)-2-amino-3-methylpentanoic acid | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.726 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H13NO2 | |||
| Molar mass | 131.175 g·mol−1 | ||
| −84.9·10−6 cm3/mol | |||
| Supplementary data page | |||
| Isoleucine (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Metabolism
Biosynthesis
In plants and microorganisms, isoleucine is synthesized via several steps starting from pyruvate and alpha-ketobutyrate. As an essential nutrient, this pathway is not present in humans. Enzymes involved in this biosynthesis include:[4]
- Acetolactate synthase (also known as acetohydroxy acid synthase)
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- Valine aminotransferase
Catabolism
Isoleucine is both a glucogenic and a ketogenic amino acid.[4] After transamination with alpha-ketoglutarate the carbon skeleton is oxidised and split into propionyl-CoA and acetyl-CoA. Propionyl-CoA is converted into succinyl-CoA, a TCA cycle intermediate which can be converted into oxaloacetate for gluconeogenesis (hence glucogenic). In mammals acetyl-CoA cannot be converted to carbohydrate but can be either fed into the TCA cycle by condensing with oxaloacetate to form citrate or used in the synthesis of ketone bodies (hence ketogenic) or fatty acids.[5]
Metabolic diseases
The degradation of isoleucine is impaired in the following metabolic diseases:
Insulin resistance
Isoleucine, like other branched-chain amino acids, is associated with insulin resistance: higher levels of isoleucine are observed in the blood of diabetic mice, rats, and humans.[6] Mice fed an isoleucine deprivation diet for one day have improved insulin sensitivity, and feeding of an isoleucine deprivation diet for one week significantly decreases blood glucose levels.[7] In diet-induced obese and insulin resistant mice, a diet with decreased levels of isoleucine (with or without the other branched-chain amino acids) results in reduced adiposity and improved insulin sensitivity.[8][9] Reduced dietary levels of isoleucine are required for the beneficial metabolic effects of a low protein diet.[9] In humans, a protein restricted diet lowers blood levels of isoleucine and decreases fasting blood glucose levels.[10] In humans, higher dietary levels of isoleucine are associated with greater body mass index.[9]
Functions and requirement
The Food and Nutrition Board (FNB) of the U.S. Institute of Medicine has set Recommended Dietary Allowances (RDAs) for essential amino acids in 2002. For adults 19 years and older, 19 mg of isoleucine/kg body weight is required daily.[11]
Beside its biological role as a nutrient, isoleucine also has been shown to participate in regulation of glucose metabolism.[12] Isoleucine is an essential component of many proteins. As an essential amino acid, isoleucine must be ingested or protein production in the cell will be disrupted. Fetal hemoglobin is one of the many proteins that require isoleucine.[13] Isoleucine is present in the gamma chain of fetal hemoglobin and must be present for the protein to form. [13]
There are genetic diseases that change the consumption requirements of isoleucine. Amino acids cannot be stored in the body. Buildup of excess amino acids will cause a buildup of toxic molecules so, humans have many pathways to degrade each amino acid when the need for protein synthesis has been met.[14] Mutations in isoleucine-degrading enzymes can lead to dangerous buildup of isoleucine and it's toxic derivative. One example is maple syrup urine disease (MSUD), a disorder that leaves people unable to breakdown isoleucine, valine, and leucine.[15] People with MSUD manage their disease by a reduced intake of all three of those amino acids alongside drugs that help excrete built-up toxins. [16]
Nutritional sources
There are many animal and plant-based dietary sources of isoleucine. Isoleucine is commonly ingested as a component of proteins. When proteins enter the small intestine they are broken into single amino acids by enzymes known as proteases.[4] Free amino acids are taken up by the lining of the digestive tract, mainly in the small intestine, then used in the body.[17] As an essential component of many proteins, animal and plant based protein sources contain isoleucine.[12] Foods that have high amounts of isoleucine include eggs, soy protein, seaweed, turkey, chicken, lamb, cheese, and fish.
Synthesis
There are many possible routes to synthesize isoleucine. One common multistep procedure starts from 2-bromobutane and diethylmalonate.[18] Synthetic isoleucine was first reported in 1905 by French chemists Bouveault and Locquin.[19]
Discovery
German chemist Felix Ehrlich discovered isoleucine while studying the composition of beet-sugar molasses 1903.[20] In 1907 Ehrlich carried out further studies on fibrin, egg albumin, gluten, and beef muscle in 1907. These studies verified the natural composition of isoleucine.[20] Ehrlich published his own synthesis of isoleucine in 1908. [21]
See also
- Alloisoleucine, the diasteromer of isoleucine
References
- "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature and symbolism for amino acids and peptides. Recommendations 1983". Biochemical Journal. 219 (2): 345–373. 1984-04-15. doi:10.1042/bj2190345. ISSN 0264-6021. PMC 1153490. PMID 6743224.
- Joshi, Vijay; Joung, Je-Gun; Fei, Zhangjun; Jander, Georg (2010-10-01). "Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress". Amino Acids. 39 (4): 933–947. doi:10.1007/s00726-010-0505-7. ISSN 1438-2199. PMID 20186554. S2CID 22641155.
- Kisumi, M.; Komatsubara, S.; Chibata, I. (1977). "Pathway for isoleucine formation form pyruvate by leucine biosynthetic enzymes in leucine-accumulating isoleucine revertants of Serratia marcescens". Journal of Biochemistry. 82 (1): 95–103. doi:10.1093/oxfordjournals.jbchem.a131698. ISSN 0021-924X. PMID 142769.
- Lehninger, Albert L. (2000). Lehninger principles of biochemistry. David L. Nelson, Michael M. Cox (3rd ed.). New York: Worth Publishers. ISBN 1-57259-153-6. OCLC 42619569.
- Branched chain amino acids in clinical nutrition. Volume 1. Rajkumar Rajendram, Victor R. Preedy, Vinood B. Patel. New York, New York. 2015. ISBN 978-1-4939-1923-9. OCLC 898999904.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - Lynch, Christopher J.; Adams, Sean H. (2014-10-07). "Branched-chain amino acids in metabolic signalling and insulin resistance". Nature Reviews Endocrinology. 10 (12): 723–736. doi:10.1038/nrendo.2014.171. ISSN 1759-5029. PMC 4424797. PMID 25287287.
- Xiao, Fei; Yu, Junjie; Guo, Yajie; Deng, Jiali; Li, Kai; Du, Ying; Chen, Shanghai; Zhu, Jianmin; Sheng, Hongguang; Guo, Feifan (2014). "Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice". Metabolism. 63 (6): 841–850. doi:10.1016/j.metabol.2014.03.006. ISSN 0026-0495. PMID 24684822.
- Cummings, Nicole E.; Williams, Elizabeth M.; Kasza, Ildiko; Konon, Elizabeth N.; Schaid, Michael D.; Schmidt, Brian A.; Poudel, Chetan; Sherman, Dawn S.; Yu, Deyang; Arriola Apelo, Sebastian I.; Cottrell, Sara E.; Geiger, Gabriella; Barnes, Macy E.; Wisinski, Jaclyn A.; Fenske, Rachel J. (2018-02-15). "Restoration of metabolic health by decreased consumption of branched-chain amino acids". The Journal of Physiology. 596 (4): 623–645. doi:10.1113/JP275075. ISSN 1469-7793. PMC 5813603. PMID 29266268.
- Yu, Deyang; Richardson, Nicole E.; Green, Cara L.; Spicer, Alexandra B.; Murphy, Michaela E.; Flores, Victoria; Jang, Cholsoon; Kasza, Ildiko; Nikodemova, Maria; Wakai, Matthew H.; Tomasiewicz, Jay L.; Yang, Shany E.; Miller, Blake R.; Pak, Heidi H.; Brinkman, Jacqueline A. (2021-05-04). "The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine". Cell Metabolism. 33 (5): 905–922.e6. doi:10.1016/j.cmet.2021.03.025. ISSN 1932-7420. PMC 8102360. PMID 33887198.
- Fontana, Luigi; Cummings, Nicole E.; Arriola Apelo, Sebastian I.; Neuman, Joshua C.; Kasza, Ildiko; Schmidt, Brian A.; Cava, Edda; Spelta, Francesco; Tosti, Valeria; Syed, Faizan A.; Baar, Emma L.; Veronese, Nicola; Cottrell, Sara E.; Fenske, Rachel J.; Bertozzi, Beatrice (2016-07-12). "Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health". Cell Reports. 16 (2): 520–530. doi:10.1016/j.celrep.2016.05.092. ISSN 2211-1247. PMC 4947548. PMID 27346343.
- Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Institute of Medicine. Panel on Macronutrients, Institute of Medicine. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington, D.C.: National Academies Press. 2005. ISBN 0-309-08537-3. OCLC 57373786.
{{cite book}}: CS1 maint: others (link) - Branched chain amino acids in clinical nutrition. Volume 1. Rajkumar Rajendram, Victor R. Preedy, Vinood B. Patel. New York, New York. 2015. ISBN 978-1-4939-1923-9. OCLC 898999904.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - Honig, George R. (1967-11-01). "Inhibition of Synthesis of Fetal Hemoglobin by an Isoleucine Analogue". The Journal of Clinical Investigation. 46 (11): 1778–1784. doi:10.1172/JCI105668. ISSN 0021-9738. PMC 292928. PMID 4964832.
- Korman, Stanley H. (2006-12-01). "Inborn errors of isoleucine degradation: A review". Molecular Genetics and Metabolism. 89 (4): 289–299. doi:10.1016/j.ymgme.2006.07.010. ISSN 1096-7192. PMID 16950638.
- Hassan, Syed Adeel; Gupta, Vikas (2023), "Maple Syrup Urine Disease", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 32491705, retrieved 2023-04-16
- Brunetti-Pierri, Nicola; Lanpher, Brendan; Erez, Ayelet; Ananieva, Elitsa A.; Islam, Mohammad; Marini, Juan C.; Sun, Qin; Yu, Chunli; Hegde, Madhuri; Li, Jun; Wynn, R. Max; Chuang, David T.; Hutson, Susan; Lee, Brendan (2011-02-15). "Phenylbutyrate therapy for maple syrup urine disease". Human Molecular Genetics. 20 (4): 631–640. doi:10.1093/hmg/ddq507. ISSN 1460-2083. PMC 3024040. PMID 21098507.
- Bröer, S.; Fairweather, S. J. (2011-01-17). Terjung, Ronald (ed.). Comprehensive Physiology. Vol. 9 (1 ed.). Wiley. pp. 343–373. doi:10.1002/cphy.c170041. ISBN 978-0-470-65071-4. PMID 30549024. S2CID 56485230.
- "dl-ISOLEUCINE". Organic Syntheses. 21: 60. 1941. doi:10.15227/orgsyn.021.0060. ISSN 0078-6209.
- Bouvealt, L; Locquin, R (1905). "Sur la synthése d'une nouvelle leucine". Compt. Rend. (141): 115–117.
- Vickery, Hubert Bradford.; Schmidt, Carl L. A. (1931-10-01). "The History of the Discovery of the Amino Acids". Chemical Reviews. 9 (2): 169–318. doi:10.1021/cr60033a001. ISSN 0009-2665.
- Ehrlich, Felix (1908). "Über eine Synthese des Isoleucins". Chemische Berichte. 41 (1): 1453–1458. doi:10.1002/cber.190804101266. ISSN 0365-9496.