List of esters
In chemistry, an ester is a compound derived from an acid (organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group (−OH) of that acid is replaced by an organyl group (−R). Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well (i.e. esters of acidic −SH, −SeH, −TeH, −PoH and −LvH groups). According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC.[1]
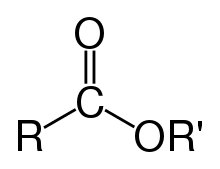
An example of an ester formation is the substitution reaction between a carboxylic acid (R−C(=O)−OH) and an alcohol (R'OH), forming an ester (R−C(=O)−O−R'), where R and R′ are organyl groups, or H in the case of esters of formic acid. Glycerides, which are fatty acid esters of glycerol, are important esters in biology, being one of the main classes of lipids, and making up the bulk of animal fats and vegetable oils. Esters of carboxylic acids with low molecular weight are commonly used as fragrances and found in essential oils and pheromones. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties, while polyesters are important plastics, with monomers linked by ester moieties. Esters of carboxylic acids usually have a sweet smell and are considered high-quality solvents for a broad array of plastics, plasticizers, resins, and lacquers.[2] They are also one of the largest classes of synthetic lubricants on the commercial market.[3]
By number of alkyl group carbons
1 carbon
2 carbons
3 carbons
| Name | Structure |
|---|---|
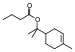
| Propyl acetate |  |
| Propyl propanoate | |
| Propyl hexanoate | |
| Allyl hexanoate | |
| Isopropyl acetate | 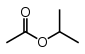 |
| Isopropyl salicylate | 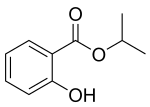 |
| Isopropyl palmitate |
4 carbons
| Name | Structure |
|---|---|
| Butyl formate | |
| Butyl acetate | |
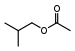
| Isobutyl formate | |
| Isobutyl acetate |  |
| Sec-Butyl formate | |
| Sec-Butyl acetate | |
| Tert-Butyl formate | |
| Tert-Butyl acetate | 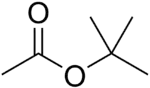 |
| Butyl butyrate |
5 carbons
| Name | Structure |
|---|---|
| Amyl acetate | |
| Pentyl butyrate | |
| Pentyl propanoate | |
| Pentyl hexanoate | |
| Sec-Amyl acetate |
7 carbons
| Name | Structure |
|---|---|
| Benzyl acetate | 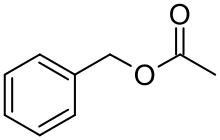 |
8 carbons
| Name | Structure |
|---|---|
| Octyl acetate |
10 carbons
| Name | Structure |
|---|---|
| Geranyl acetate | |
| Bornyl acetate | 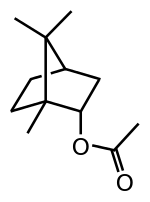 |
| Linalyl acetate | 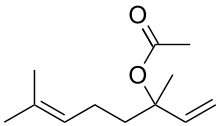 |
By number of acid group carbons
0 carbons
| Name | Structure |
|---|---|
| Methyl nitrate | 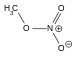 |
1 carbon
| Name | Structure |
|---|---|
| Methyl formate | |
| Ethyl formate | |
| Isobutyl formate |
2 carbons
| Name | Structure |
|---|---|
| Methyl acetate | 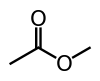 |
| Ethyl acetate | 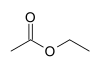 |
| Propyl acetate | |
| Butyl acetate | |
| Amyl acetate | |
| Benzyl acetate |  |
| Octyl acetate | |
| Geranyl acetate | |
| Bornyl acetate |  |
| Linalyl acetate |  |
3 carbons
| Name | Structure |
|---|---|
| Methyl propionate | |
| Ethyl propionate | |
| Propyl propanoate | |
| Pentyl propanoate | |
| Ethyl lactate | 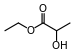 |
4 carbons
| Name | Structure |
|---|---|
| Methyl butyrate | |
| Ethyl butyrate | |
| Butyl butyrate | |
| Pentyl butyrate |
5 carbons
| Name | Structure |
|---|---|
| Methyl pentanoate | |
| Ethyl pentanoate | |
| Pentyl pentanoate | |
| Ethyl isovalerate |
6 carbons
| Name | Structure |
|---|---|
| Ethyl hexanoate | |
| Propyl hexanoate | |
| Allyl hexanoate | |
| Pentyl hexanoate |
7 carbons
| Name | Structure |
|---|---|
| Ethyl heptanoate | |
| Methyl benzoate | 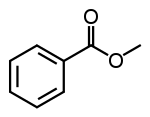 |
| Ethyl benzoate | 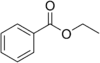 |
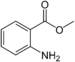
| Methyl anthranilate | 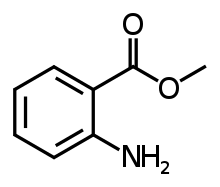 |
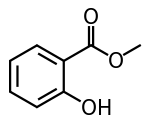
| Methyl salicylate | 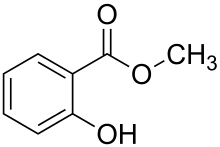 |
| Ethyl salicylate | 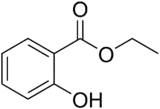 |
| Isopropyl salicylate | 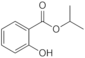 |
8 carbons
| Name | Structure |
|---|---|
| Ethyl octanoate | |
| Methyl phenylacetate | 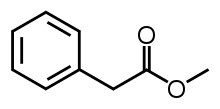 |
9 carbons
| Name | Structure |
|---|---|
| Methyl cinnamate | 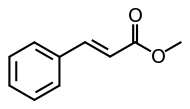 |
| Ethyl cinnamate | 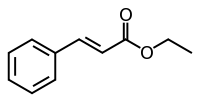 |
10 carbons
| Name | Structure |
|---|---|
| Ethyl decanoate |
16 carbons
| Name | Structure |
|---|---|
| Isopropyl palmitate |
List of ester odorants
Many esters of carboxylic acid have distinctive fruit-like odors, and many occur naturally in fruits and the essential oils of plants. This has also led to their common use in artificial flavorings and fragrances which aim to mimic those odors.
Lactones
Lactones are a specific class cyclic carboxylic esters that are formed through intramolecular esterification.
| Lactone name | Structure |
|---|---|
| β-propiolactone | 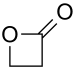 |
| γ-butyrolactone (GBL) | -one_200.svg.png.webp) |
| D-glucono-δ-lactone (E575) | 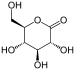 |
| ε-caprolactone | 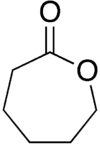 |
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "esters". doi:10.1351/goldbook.E02219
- Cameron Wright (1986). A worker's guide to solvent hazards. The Group. p. 48. ISBN 9780969054542.
- E. Richard Booser (21 December 1993). CRC Handbook of Lubrication and Tribology, Volume III: Monitoring, Materials, Synthetic Lubricants, and Applications. CRC. p. 237. ISBN 978-1-4200-5045-5.
- Reichel, Marco; Krumm, Burkhard; Vishnevskiy, Yury V.; Blomeyer, Sebastian; Schwabedissen, Jan; Stammler, Hans‐Georg; Karaghiosoff, Konstantin; Mitzel, Norbert W. (2019-12-16). "Solid‐State and Gas‐Phase Structures and Energetic Properties of the Dangerous Methyl and Fluoromethyl Nitrates". Angewandte Chemie International Edition. 58 (51): 18557–18561. doi:10.1002/anie.201911300. ISSN 1433-7851. PMC 6916544. PMID 31573130.
- Werner Reutemann and Heinz Kieczka "Formic Acid" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a12_013
- Merck Index, 12th Edition, 6089.
- "Methyl Propionate Hazardous Substance Fact Sheet" (PDF). New Jersey Department of Health and Senior Services.
- Methyl butyrate, thegoodscentscompany.com
- Methyl pentanoate, thegoodscentscompany.com
- Methyl benzoate, thegoodscentscompany.com
- Methyl anthranilate, thegoodscentscompany.com
- Methyl salicylate, thegoodscentscompany.com
- Methyl phenylacetate, thegoodscentscompany.com
- Methyl cinnamate, thegoodscentscompany.com
- Ethyl formate, thegoodscentscompany.com
- Ethyl acetate, thegoodscentscompany.com
- Ethyl propionate, thegoodscentscompany.com
- Ethyl lactate, thegoodscentscompany.com
- Ethyl butyrate, thegoodscentscompany.com
- Ethyl pentanoate, thegoodscentscompany.com
- Ethyl isovalerate, thegoodscentscompany.com
- Ethyl hexanoate, thegoodscentscompany.com
- Ethyl heptanoate, thegoodscentscompany.com
- Ethyl benzoate, thegoodscentscompany.com
- Ethyl salicylate, thegoodscentscompany.com
- Ethyl octanoate, thegoodscentscompany.com
- Ethyl cinnamate, thegoodscentscompany.com
- Ethyl decanoate, thegoodscentscompany.com