Lutetium(III) chloride
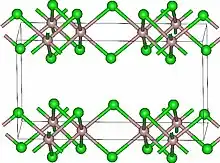
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic white monoclinic crystals[1] and also a hydroscopic hexahydrate LuCl3·6H2O.[6] Anhydrous lutetium(III) chloride has the YCl3 (AlCl3) layer structure with octahedral lutetium ions.[7]
 | |
| Names | |
|---|---|
| IUPAC name
Lutetium(III) chloride | |
| Other names
Lutetium chloride, lutetium trichloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.205 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| LuCl3 | |
| Molar mass | 281.325 g/mol |
| Appearance | colorless or white monoclinic crystals |
| Density | 3.98 g/cm3 |
| Melting point | 905 °C (1,661 °F; 1,178 K)[1] |
| Boiling point | sublimes above 750°C[2] |
| soluble[3] | |
| Structure | |
| Monoclinic, mS16 | |
| C2/m, No. 12 | |
| Pharmacology | |
| License data | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling:[4][5] | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions |
Lutetium(III) oxide |
Other cations |
Ytterbium(III) chloride Scandium(III) chloride Yttrium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Reactions
Pure lutetium metal can be produced from lutetium(III) chloride by heating it together with elemental calcium:[8]
See also
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, p. 472, ISBN 0-8493-0594-2, retrieved 2008-06-27
- "Chemistry: Periodic Table: Lutetium: compound data (lutetium (III) chloride)". WebElements. Retrieved 2008-06-27.
- Perry, Dale L.; Phillips, Sidney L. (1995), Handbook of Inorganic Compounds, CRC Press, p. 232, ISBN 0-8493-8671-3, retrieved 2008-06-27
- "450960 Lutetium(III) chloride anhydrous, powder, 99.99% trace metals basis". Sigma-Aldrich. Retrieved 2008-06-27.
- "Lutetium chloride". pubchem.ncbi.nlm.nih.gov.
- "Lutetium(III) chloride hexahydrate 542075". Sigma-Aldrich. Retrieved 2019-07-24.
- Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- Patnaik, Pradyot (2004), Handbook of Inorganic Chemicals, Amsterdam: McGraw-Hill Professional, p. 244, ISBN 0-07-049439-8, retrieved 2008-06-27
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
