Extinction event
An extinction event (also known as a mass extinction or biotic crisis) is a widespread and rapid decrease in the biodiversity on Earth. Such an event is identified by a sharp change in the diversity and abundance of multicellular organisms. It occurs when the rate of extinction increases with respect to the background extinction rate[1] and the rate of speciation. Estimates of the number of major mass extinctions in the last 540 million years range from as few as five to more than twenty. These differences stem from disagreement as to what constitutes a "major" extinction event, and the data chosen to measure past diversity.
The "Big Five" mass extinctions
In a landmark paper published in 1982, Jack Sepkoski and David M. Raup identified five particular geological intervals with excessive diversity loss.[2] They were originally identified as outliers on a general trend of decreasing extinction rates during the Phanerozoic,[3] but as more stringent statistical tests have been applied to the accumulating data, it has been established that multicellular animal life has experienced at least five major and many minor mass extinctions.[4] The "Big Five" cannot be so clearly defined, but rather appear to represent the largest (or some of the largest) of a relatively smooth continuum of extinction events.[3] An earlier (first) event at the end of the Ediacaran is speculated.[5]
- Ordovician–Silurian extinction events (End Ordovician or O–S): 445–444 Ma, just prior to and at the Ordovician–Silurian transition. Two events occurred that killed off 27% of all families, 57% of all genera and 85% of all species.[6] Together they are ranked by many scientists as the second-largest of the five major extinctions in Earth's history in terms of percentage of genera that became extinct. In May 2020, studies suggested that the causes of the mass extinction were global warming, related to volcanism, and anoxia, and not, as considered earlier, cooling and glaciation.[7][8] However, this is at odds with numerous previous studies, which have indicated global cooling as the primary driver.[9] Most recently, the deposition of volcanic ash has been suggested to be the trigger for reductions in atmospheric carbon dioxide leading to the glaciation and anoxia observed in the geological record.[10]
- Late Devonian extinctions: 372–359 Ma, occupying much of the Late Devonian up to the Devonian–Carboniferous transition. The Late Devonian was an interval of high diversity loss, concentrated into two extinction events. The largest extinction was the Kellwasser Event (Frasnian-Famennian, or F-F, 372 Ma), an extinction event at the end of the Frasnian, about midway through the Late Devonian. This extinction annihilated coral reefs and numerous tropical benthic (seabed-living) animals such as jawless fish, brachiopods, and trilobites. Another major extinction was the Hangenberg Event (Devonian-Carboniferous, or D-C, 359 Ma), which brought an end to the Devonian as a whole. This extinction wiped out the armored placoderm fish and nearly led to the extinction of the newly evolved ammonoids. These two closely-spaced extinction events collectively eliminated about 19% of all families, 50% of all genera[6] and at least 70% of all species.[11] Sepkoski and Raup (1982) did not initially consider the Late Devonian extinction interval (Givetian, Frasnian, and Famennian stages) to be statistically significant.[2] Regardless, later studies have affirmed the strong ecological impacts of the Kellwasser and Hangenberg Events.[12]
- Permian–Triassic extinction event (End Permian): 252 Ma, at the Permian–Triassic transition.[13] Earth's largest extinction killed 53% of marine families, 84% of marine genera, about 81% of all marine species[14] and an estimated 70% of terrestrial vertebrate species.[15] This is also the largest known extinction event for insects.[16] The highly successful marine arthropod, the trilobite, became extinct. The evidence regarding plants is less clear, but new taxa became dominant after the extinction.[17] The "Great Dying" had enormous evolutionary significance: On land, it ended the primacy of early synapsids. The recovery of vertebrates took 30 million years,[18] but the vacant niches created the opportunity for archosaurs to become ascendant. In the seas, the percentage of animals that were sessile (unable to move about) dropped from 67% to 50%. The whole late Permian was a difficult time, at least for marine life, even before the P–T boundary extinction. More recent research has indicated that the End-Capitanian extinction event that preceded the "Great Dying" likely constitutes a separate event from the P–T extinction; if so, it would be larger than some of the "Big Five" extinction events, and perhaps merit a separate place in this list immediately before this one.
 Trilobites were highly successful marine animals until the Permian–Triassic extinction event wiped them all out.
Trilobites were highly successful marine animals until the Permian–Triassic extinction event wiped them all out. - Triassic–Jurassic extinction event (End Triassic): 201.3 Ma, at the Triassic–Jurassic transition. About 23% of all families, 48% of all genera (20% of marine families and 55% of marine genera) and 70% to 75% of all species became extinct.[6] Most non-dinosaurian archosaurs, most therapsids, and most of the large amphibians were eliminated, leaving dinosaurs with little terrestrial competition. Non-dinosaurian archosaurs continued to dominate aquatic environments, while non-archosaurian diapsids continued to dominate marine environments. The Temnospondyl lineage of large amphibians also survived until the Cretaceous in Australia (e.g., Koolasuchus).
- Cretaceous–Paleogene extinction event (End Cretaceous, K–Pg extinction, or formerly K–T extinction): 66 Ma, at the Cretaceous (Maastrichtian) – Paleogene (Danian) transition.[19] The event was formerly called the Cretaceous-Tertiary or K–T extinction or K–T boundary; it is now officially named the Cretaceous–Paleogene (or K–Pg) extinction event. About 17% of all families, 50% of all genera[6] and 75% of all species became extinct.[2] In the seas all the ammonites, plesiosaurs and mosasaurs disappeared and the percentage of sessile animals was reduced to about 33%. All non-avian dinosaurs became extinct during that time.[20] The boundary event was severe with a significant amount of variability in the rate of extinction between and among different clades. Mammals and birds, the former descended from the synapsids and the latter from theropod dinosaurs, emerged as dominant terrestrial animals.

Despite the popularization of these five events, there is no definite line separating them from other extinction events; using different methods of calculating an extinction's impact can lead to other events featuring in the top five.[21]
Older fossil records are more difficult to interpret. This is because:
- Older fossils are harder to find as they are usually buried at a considerable depth.
- Dating of older fossils is more difficult.
- Productive fossil beds are researched more than unproductive ones, therefore leaving certain periods unresearched.
- Prehistoric environmental events can disturb the deposition process.
- The preservation of fossils varies on land, but marine fossils tend to be better preserved than their sought after land-based counterparts.[22]
It has been suggested that the apparent variations in marine biodiversity may actually be an artifact, with abundance estimates directly related to quantity of rock available for sampling from different time periods.[23] However, statistical analysis shows that this can only account for 50% of the observed pattern, and other evidence such as fungal spikes (geologically rapid increase in fungal abundance) provides reassurance that most widely accepted extinction events are real. A quantification of the rock exposure of Western Europe indicates that many of the minor events for which a biological explanation has been sought are most readily explained by sampling bias.[24]
Sixth mass extinction
Research completed after the seminal 1982 paper (Sepkoski and Raup) has concluded that a sixth mass extinction event is ongoing due to human activities:
- Holocene extinction: currently ongoing. Extinctions have occurred at over 1000 times the background extinction rate since 1900, and the rate is increasing.[25][26][lower-alpha 1] The mass extinction is a result of human activity (an ecocide)[28][29][30][31] driven by population growth and overconsumption of the earth's natural resources.[lower-alpha 2] The 2019 global biodiversity assessment by IPBES asserts that out of an estimated 8 million species, 1 million plant and animal species are currently threatened with extinction.[33][34][35][36] In late 2021, WWF Germany suggested that over a million species could go extinct within a decade in the "largest mass extinction event since the end of the dinosaur age."[37] A 2023 study published in PNAS concluded that at least 73 genera of animals have gone extinct since 1500. If humans had never existed, it would have taken 18,000 years for the same genera to have disappeared naturally, the report states.[38][39][40]
Extinctions by severity
Extinction events can be tracked by several methods, including geological change, ecological impact, extinction vs. origination (speciation) rates, and most commonly diversity loss among taxonomic units. Most early papers used families as the unit of taxonomy, based on compendiums of marine animal families by Sepkoski (1982, 1992).[41][42] Later papers by Sepkoski and other authors switched to genera, which are more precise than families and less prone to taxonomic bias or incomplete sampling relative to species.[43] These are several major papers estimating loss or ecological impact from fifteen commonly-discussed extinction events. Different methods used by these papers are described in the following section. The "Big Five" mass extinctions are bolded.
| Extinction name | Age (Ma) |
Sepkoski (1996)[44] Multiple-interval genera |
Bambach (2006)[45] |
McGhee et al. (2013)[12] | Stanley (2016)[14] | |
|---|---|---|---|---|---|---|
| Taxonomic loss |
Ecological ranking | |||||
| Late Ordovician (Ashgillian / Hirnantian) | 445-444 | ~49% | 57%[d] (40%, 31%)[e] |
52% | 7 | 42-46% |
| Lau event (Ludfordian) | 424 | ~23% | - | 9% | 9 | - |
| Kačák Event (Eifelian) | ~388 | ~24%[a] | - | 32% | 9 | - |
| Taghanic Event (Givetian) | ~384 | ~30%[a] | 28.5% | 36% | 8 | - |
| Late Devonian/Kellwasser event (Frasnian) | 372 | ~35% | 34.7% | 40% | 4 | 16-20% |
| End-Devonian/Hangenberg event (Famennian) | 359 | ~28%[a] | 31% | 50% | 7 | <13%[f] |
| Serpukhovian | ~330-325 | ~23% | 31% | 39% | 6 | 13-15% |
| Capitanian | 260 | ~47%[b] | 48% | 25% | 5 | 33-35% |
| Permian–Triassic (Changhsingian) | 252 | ~58% | 55.7% | 83% | 1 | 62% |
| Triassic–Jurassic (Rhaetian) | 201 | ~37%[c] | 47%[c] | 73% | 3 | N/A[g] |
| Pliensbachian-Toarcian | 186-178 | ~14% | 25%, 20%[e] | - | - | - |
| End-Jurassic (Tithonian) | 145 | ~18% | 20% | - | - | - |
| Cenomanian-Turonian | 94 | ~15% | 25% | - | - | - |
| Cretaceous–Paleogene (Maastrichtian) | 66 | ~39% | 40-47% | 40% | 2 | 38-40% |
| Eocene–Oligocene | 34 | ~11% | 15.6% | - | - | - |
a Graphed but not discussed by Sepkoski (1996), considered continuous with the Late Devonian mass extinction
b At the time considered continuous with the end-Permian mass extinction
c Includes late Norian time slices
d Diversity loss of both pulses calculated together
e Pulses extend over adjacent time slices, calculated separately
f Considered ecologically significant, but not analyzed directly
g Excluded due to a lack of consensus on Late Triassic chronology
The study of major extinction events
Breakthrough studies in the 1980s–1990s

For much of the 20th century, the study of mass extinctions was hampered by insufficient data. Mass extinctions, though acknowledged, were considered mysterious exceptions to the prevailing gradualistic view of prehistory, where slow evolutionary trends define faunal changes. The first breakthrough was published in 1980 by a team led by Luis Alvarez, who discovered trace metal evidence for an asteroid impact at the end of the Cretaceous period. The Alvarez hypothesis for the end-Cretaceous extinction gave mass extinctions, and catastrophic explanations, newfound popular and scientific attention.[46]
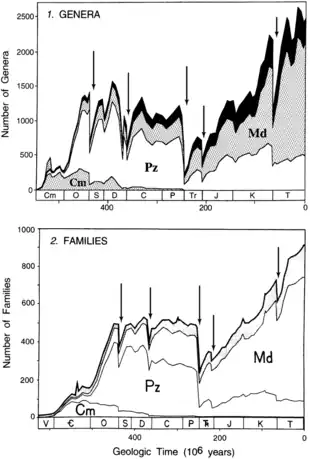
Another landmark study came in 1982, when a paper written by David M. Raup and Jack Sepkoski was published in the journal Science.[2] This paper, originating from a compendium of extinct marine animal families developed by Sepkoski,[41] identified five peaks of marine family extinctions which stand out among a backdrop of decreasing extinction rates through time. Four of these peaks were statistically significant: the Ashgillian (end-Ordovician), Late Permian, Norian (end-Triassic), and Maastrichtian (end-Cretaceous). The remaining peak was a broad interval of high extinction smeared over the later half of the Devonian, with its apex in the Frasnian stage.[2]
Through the 1980s, Raup and Sepkoski continued to elaborate and build upon their extinction and origination data, defining a high-resolution biodiversity curve (the "Sepkoski curve") and successive evolutionary faunas with their own patterns of diversification and extinction.[47][48][49][50][51][52] Though these interpretations formed a strong basis for subsequent studies of mass extinctions, Raup and Sepkoski also proposed a more controversial idea in 1984: a 26-million-year periodic pattern to mass extinctions.[53] Two teams of astronomers linked this to a hypothetical brown dwarf in the distant reaches of the solar system, inventing the "Nemesis hypothesis" which has been strongly disputed by other astronomers.
Around the same time, Sepkoski began to devise a compendium of marine animal genera, which would allow researchers to explore extinction at a finer taxonomic resolution. He began to publish preliminary results of this in-progress study as early as 1986, in a paper which identified 29 extinction intervals of note.[51] By 1992, he also updated his 1982 family compendium, finding minimal changes to the diversity curve despite a decade of new data.[42][54] In 1996, Sepkoski published another paper which tracked marine genera extinction (in terms of net diversity loss) by stage, similar to his previous work on family extinctions. The paper filtered its sample in three ways: all genera (the entire unfiltered sample size), multiple-interval genera (only those found in more than one stage), and "well-preserved" genera (excluding those from groups with poor or understudied fossil records). Diversity trends in marine animal families were also revised based on his 1992 update.[44]
Revived interest in mass extinctions led many other authors to re-evaluate geological events in the context of their effects on life.[55] A 1995 paper by Michael Benton tracked extinction and origination rates among both marine and continental (freshwater & terrestrial) families, identifying 22 extinction intervals and no periodic pattern.[56] Overview books by O.H. Wallister (1996) and A. Hallam and P.B. Wignall (1997) summarized the new extinction research of the previous two decades.[57][58] One chapter in the former source lists over 60 geological events which could conceivably be considered global extinctions of varying sizes.[59] These texts, and other widely circulated publications in the 1990s, helped to establish the popular image of mass extinctions as a "big five" alongside many smaller extinctions through prehistory.
New data on genera: Sepkoski's compendium
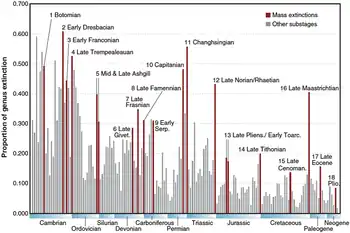
Though Sepkoski passed away in 1999, his marine genera compendium was formally published in 2002. This prompted a new wave of studies into the dynamics of mass extinctions.[43] These papers utilized the compendium to track origination rates (the rate that new species appear or speciate) parallel to extinction rates in the context of geological stages or substages.[60] A review and re-analysis of Sepkoski's data by Bambach (2006) identified 18 distinct mass extinction intervals, including 4 large extinctions in the Cambrian. These fit Sepkoski's definition of extinction, as short substages with large diversity loss and overall high extinction rates relative to their surroundings.[45]
Bambach et al. (2004) considered each of the "Big Five" extinction intervals to have a different pattern in the relationship between origination and extinction trends. Moreover, background extinction rates were broadly variable and could be separated into more severe and less severe time intervals. Background extinctions were least severe relative to the origination rate in the middle Ordovician-early Silurian, late Carboniferous-Permian, and Jurassic-recent. This argues that the Late Ordovician, end-Permian, and end-Cretaceous extinctions were statistically significant outliers in biodiversity trends, while the Late Devonian and end-Triassic extinctions occurred in time periods which were already stressed by relatively high extinction and low origination.[61]
Computer models run by Foote (2005) determined that abrupt pulses of extinction fit the pattern of prehistoric biodiversity much better than a gradual and continuous background extinction rate with smooth peaks and troughs. This strongly supports the utility of rapid, frequent mass extinctions as a major driver of diversity changes. Pulsed origination events are also supported, though to a lesser degree which is largely dependent on pulsed extinctions.[62]
Similarly, Stanley (2007) used extinction and origination data to investigate turnover rates and extinction responses among different evolutionary faunas and taxonomic groups. In contrast to previous authors, his diversity simulations show support for an overall exponential rate of biodiversity growth through the entire Phanerozoic.[63]
Tackling biases in the fossil record
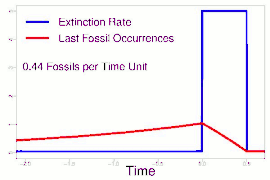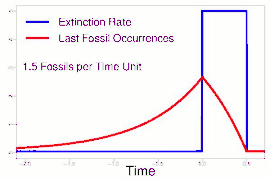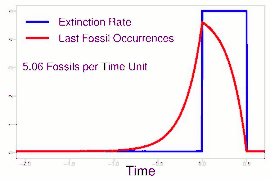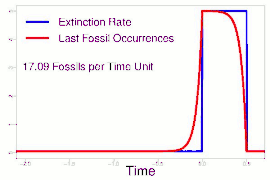
As data continued to accumulate, some authors began to re-evaluate Sepkoski's sample using methods meant to account for sampling biases. As early as 1982, a paper by Phillip W. Signor and Jere H. Lipps noted that the true sharpness of extinctions was diluted by the incompleteness of the fossil record.[64] This phenomenon, later called the Signor-Lipps effect, notes that a species' true extinction must occur after its last fossil, and that origination must occur before its first fossil. Thus, species which appear to die out just prior to an abrupt extinction event may instead be a victim of the event, despite an apparent gradual decline looking at the fossil record alone. A model by Foote (2007) found that many geological stages had artificially inflated extinction rates due to Signor-Lipps "backsmearing" from later stages with extinction events.[65]
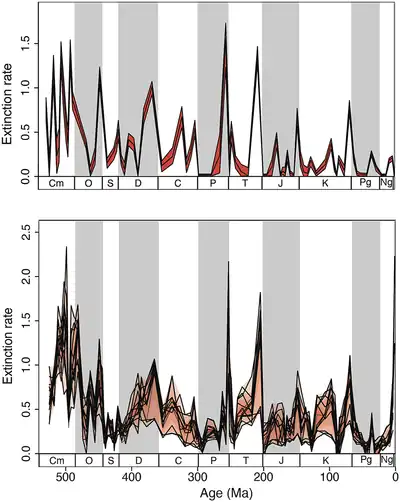
Other biases include the difficulty in assessing taxa with high turnover rates or restricted occurrences, which cannot be directly assessed due to a lack of fine-scale temporal resolution. Many paleontologists opt to assess diversity trends by randomized sampling and rarefaction of fossil abundances rather than raw temporal range data, in order to account for all of these biases. But that solution is influenced by biases related to sample size. One major bias in particular is the "Pull of the recent", the fact that the fossil record (and thus known diversity) generally improves closer to the modern day. This means that biodiversity and abundance for older geological periods may be underestimated from raw data alone.[60][65][3]
Alroy (2010) attempted to circumvene sample size-related biases in diversity estimates using a method he called "shareholder quorum subsampling" (SQS). In this method, fossils are sampled from a "collection" (such as a time interval) to assess the relative diversity of that collection. Every time a new species (or other taxon) enters the sample, it brings over all other fossils belonging to that species in the collection (its "share" of the collection). For example, a skewed collection with half its fossils from one species will immediately reach a sample share of 50% if that species is the first to be sampled. This continues, adding up the sample shares until a "coverage" or "quorum" is reached, referring to a pre-set desired sum of share percentages. At that point, the number of species in the sample are counted. A collection with more species is expected to reach a sample quorum with more species, thus accurately comparing the relative diversity change between two collections without relying on the biases inherent to sample size.[66]
Alroy also elaborated on three-timer algorithms, which are meant to counteract biases in estimates of extinction and origination rates. A given taxon is a "three-timer" if it can be found before, after, and within a given time interval, and a "two-timer" if it overlaps with a time interval on one side. Counting "three-timers" and "two-timers" on either end of a time interval, and sampling time intervals in sequence, can together be combined into equations to predict extinction and origination with less bias.[66] In subsequent papers, Alroy continued to refine his equations to improve lingering issues with precision and unusual samples.[67][68]
McGhee et al. (2013), a paper which primarily focused on ecological effects of mass extinctions, also published new estimates of extinction severity based on Alroy's methods. Many extinctions were significantly more impactful under these new estimates, though some were less prominent.[12]
Stanley (2016) was another paper which attempted to remove two common errors in previous estimates of extinction severity. The first error was the unjustified removal of "singletons", genera unique to only a single time slice. Their removal would mask the influence of groups with high turnover rates or lineages cut short early in their diversification. The second error was the difficulty in distinguishing background extinctions from brief mass extinction events within the same short time interval. To circumvent this issue, background rates of diversity change (extinction/origination) were estimated for stages or substages without mass extinctions, and then assumed to apply to subsequent stages with mass extinctions. For example, the Santonian and Campanian stages were each used to estimate diversity changes in the Maastrichtian prior to the K-Pg mass extinction. Subtracting background extinctions from extinction tallies had the effect of reducing the estimated severity of the six sampled mass extinction events. This effect was stronger for mass extinctions which occurred in periods with high rates of background extinction, like the Devonian.[14]
Uncertainty in the Proterozoic and earlier eons
Because most diversity and biomass on Earth is microbial, and thus difficult to measure via fossils, extinction events placed on-record are those that affect the easily observed, biologically complex component of the biosphere rather than the total diversity and abundance of life.[69] For this reason, well-documented extinction events are confined to the Phanerozoic eon, before which all living organisms were either microbial or at most soft-bodied; the sole exception is the Great Oxidation Event in the Proterozoic. Perhaps due to the absence of a robust microbial fossil record, mass extinctions seem mainly to be a Phanerozoic phenomenon, with apparent extinction rates being low before large complex organisms arose.[70]
Extinction occurs at an uneven rate. Based on the fossil record, the background rate of extinctions on Earth is about two to five taxonomic families of marine animals every million years. Marine fossils are mostly used to measure extinction rates because of their superior fossil record and stratigraphic range compared to land animals.
The Great Oxidation Event, which occurred around 2.45 billion years ago in the Paleoproterozoic, was probably the first major extinction event.[71] Since the Cambrian explosion, five further major mass extinctions have significantly exceeded the background extinction rate. The most recent and best-known, the Cretaceous–Paleogene extinction event, which occurred approximately 66 Ma (million years ago), was a large-scale mass extinction of animal and plant species in a geologically short period of time.[72] In addition to the five major Phanerozoic mass extinctions, there are numerous minor ones as well, and the ongoing mass extinction caused by human activity is sometimes called the sixth extinction.[73]
Evolutionary importance
−4500 — – — – −4000 — – — – −3500 — – — – −3000 — – — – −2500 — – — – −2000 — – — – −1500 — – — – −1000 — – — – −500 — – — – 0 — |
| |||||||||||||||||||||||||||||||||||||||||||||
Mass extinctions have sometimes accelerated the evolution of life on Earth. When dominance of particular ecological niches passes from one group of organisms to another, it is rarely because the newly dominant group is "superior" to the old but usually because an extinction event eliminates the old, dominant group and makes way for the new one, a process known as adaptive radiation.[74][75]
For example, mammaliaformes ("almost mammals") and then mammals existed throughout the reign of the dinosaurs, but could not compete in the large terrestrial vertebrate niches that dinosaurs monopolized. The end-Cretaceous mass extinction removed the non-avian dinosaurs and made it possible for mammals to expand into the large terrestrial vertebrate niches. The dinosaurs themselves had been beneficiaries of a previous mass extinction, the end-Triassic, which eliminated most of their chief rivals, the crurotarsans.
Another point of view put forward in the Escalation hypothesis predicts that species in ecological niches with more organism-to-organism conflict will be less likely to survive extinctions. This is because the very traits that keep a species numerous and viable under fairly static conditions become a burden once population levels fall among competing organisms during the dynamics of an extinction event.
Furthermore, many groups that survive mass extinctions do not recover in numbers or diversity, and many of these go into long-term decline, and these are often referred to as "Dead Clades Walking".[76] However, clades that survive for a considerable period of time after a mass extinction, and which were reduced to only a few species, are likely to have experienced a rebound effect called the "push of the past".[77]
Darwin was firmly of the opinion that biotic interactions, such as competition for food and space – the 'struggle for existence' – were of considerably greater importance in promoting evolution and extinction than changes in the physical environment. He expressed this in The Origin of Species:
- "Species are produced and exterminated by slowly acting causes ... and the most import of all causes of organic change is one which is almost independent of altered ... physical conditions, namely the mutual relation of organism to organism – the improvement of one organism entailing the improvement or extermination of others".[78]
Patterns in frequency
Various authors have suggested that extinction events occurred periodically, every 26 to 30 million years,[79][53] or that diversity fluctuates episodically about every 62 million years.[80] Various ideas, mostly regarding astronomical influences, attempt to explain the supposed pattern, including the presence of a hypothetical companion star to the Sun,[81][82] oscillations in the galactic plane, or passage through the Milky Way's spiral arms.[83] However, other authors have concluded that the data on marine mass extinctions do not fit with the idea that mass extinctions are periodic, or that ecosystems gradually build up to a point at which a mass extinction is inevitable.[3] Many of the proposed correlations have been argued to be spurious or lacking statistical significance.[84][85][86] Others have argued that there is strong evidence supporting periodicity in a variety of records,[87] and additional evidence in the form of coincident periodic variation in nonbiological geochemical variables such as Strontium isotopes,[88] flood basalts, anoxic events, orogenies, and evaporite deposition. One explanation for this proposed cycle is carbon storage and release by oceanic crust, which exchanges carbon between the atmosphere and mantle.[89]
Mass extinctions are thought to result when a long-term stress is compounded by a short-term shock.[90] Over the course of the Phanerozoic, individual taxa appear to have become less likely to suffer extinction,[91] which may reflect more robust food webs, as well as fewer extinction-prone species, and other factors such as continental distribution.[91] However, even after accounting for sampling bias, there does appear to be a gradual decrease in extinction and origination rates during the Phanerozoic.[3] This may represent the fact that groups with higher turnover rates are more likely to become extinct by chance; or it may be an artefact of taxonomy: families tend to become more speciose, therefore less prone to extinction, over time;[3] and larger taxonomic groups (by definition) appear earlier in geological time.[92]
It has also been suggested that the oceans have gradually become more hospitable to life over the last 500 million years, and thus less vulnerable to mass extinctions,[lower-alpha 3][93][94] but susceptibility to extinction at a taxonomic level does not appear to make mass extinctions more or less probable.[91]
Causes
There is still debate about the causes of all mass extinctions. In general, large extinctions may result when a biosphere under long-term stress undergoes a short-term shock.[90] An underlying mechanism appears to be present in the correlation of extinction and origination rates to diversity. High diversity leads to a persistent increase in extinction rate; low diversity to a persistent increase in origination rate. These presumably ecologically controlled relationships likely amplify smaller perturbations (asteroid impacts, etc.) to produce the global effects observed.[3]
Identifying causes of specific mass extinctions
A good theory for a particular mass extinction should:
- explain all of the losses, not just focus on a few groups (such as dinosaurs);
- explain why particular groups of organisms died out and why others survived;
- provide mechanisms that are strong enough to cause a mass extinction but not a total extinction;
- be based on events or processes that can be shown to have happened, not just inferred from the extinction.
It may be necessary to consider combinations of causes. For example, the marine aspect of the end-Cretaceous extinction appears to have been caused by several processes that partially overlapped in time and may have had different levels of significance in different parts of the world.[95]
Arens and West (2006) proposed a "press / pulse" model in which mass extinctions generally require two types of cause: long-term pressure on the eco-system ("press") and a sudden catastrophe ("pulse") towards the end of the period of pressure.[96] Their statistical analysis of marine extinction rates throughout the Phanerozoic suggested that neither long-term pressure alone nor a catastrophe alone was sufficient to cause a significant increase in the extinction rate.
Most widely supported explanations
MacLeod (2001)[97] summarized the relationship between mass extinctions and events that are most often cited as causes of mass extinctions, using data from Courtillot, Jaeger & Yang et al. (1996),[98] Hallam (1992)[99] and Grieve & Pesonen (1992):[100]
- Flood basalt events (giant volcanic eruptions): 11 occurrences, all associated with significant extinctions[lower-alpha 4][lower-alpha 5] But Wignall (2001) concluded that only five of the major extinctions coincided with flood basalt eruptions and that the main phase of extinctions started before the eruptions.[101]
- Sea-level falls: 12, of which seven were associated with significant extinctions.[lower-alpha 5]
- Asteroid impacts: one large impact is associated with a mass extinction, that is, the Cretaceous–Paleogene extinction event; there have been many smaller impacts but they are not associated with significant extinctions,[102] or cannot be dated precisely enough. The impact that created the Siljan Ring either was just before the Late Devonian Extinction or coincided with it.[103]
The most commonly suggested causes of mass extinctions are listed below.
Flood basalt events
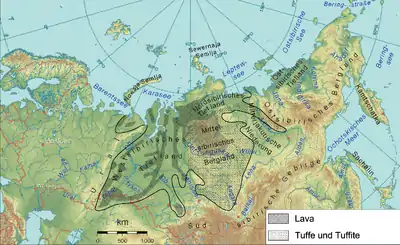
The formation of large igneous provinces by flood basalt events could have:
- produced dust and particulate aerosols, which inhibited photosynthesis and thus caused food chains to collapse both on land and at sea[104]
- emitted sulfur oxides that were precipitated as acid rain and poisoned many organisms, contributing further to the collapse of food chains
- emitted carbon dioxide and thus possibly causing sustained global warming once the dust and particulate aerosols dissipated.
Flood basalt events occur as pulses of activity punctuated by dormant periods. As a result, they are likely to cause the climate to oscillate between cooling and warming, but with an overall trend towards warming as the carbon dioxide they emit can stay in the atmosphere for hundreds of years.
Flood basalt events have been implicated as the cause of many major extinction events.[105][106] It is speculated that massive volcanism caused or contributed to the Kellwasser Event,[107][108][109] the End-Guadalupian Extinction Event,[110][111][112] the End-Permian Extinction Event,[113][114][115] the Smithian-Spathian Extinction,[116][117][118] the Triassic-Jurassic Extinction Event,[119][120][121] the Toarcian Oceanic Anoxic Event,[122][123][124] the Cenomanian-Turonian Oceanic Anoxic Event,[125][126][127] the Cretaceous-Palaeogene Extinction Event,[128][129][130] and the Palaeocene-Eocene Thermal Maximum.[131][132][133] The correlation between gigantic volcanic events expressed in the large igneous provinces and mass extinctions was shown for the last 260 million years.[134][135] Recently such possible correlation was extended across the whole Phanerozoic Eon.[136]
Sea-level fall
These are often clearly marked by worldwide sequences of contemporaneous sediments that show all or part of a transition from sea-bed to tidal zone to beach to dry land – and where there is no evidence that the rocks in the relevant areas were raised by geological processes such as orogeny. Sea-level falls could reduce the continental shelf area (the most productive part of the oceans) sufficiently to cause a marine mass extinction, and could disrupt weather patterns enough to cause extinctions on land. But sea-level falls are very probably the result of other events, such as sustained global cooling or the sinking of the mid-ocean ridges.
Sea-level falls are associated with most of the mass extinctions, including all of the "Big Five"—End-Ordovician, Late Devonian, End-Permian, End-Triassic, and End-Cretaceous, along with the more recently recognised Capitanian mass extinction of comparable severity to the Big Five.[137][138]
A 2008 study, published in the journal Nature, established a relationship between the speed of mass extinction events and changes in sea level and sediment.[139] The study suggests changes in ocean environments related to sea level exert a driving influence on rates of extinction, and generally determine the composition of life in the oceans.[140]
Impact events

The impact of a sufficiently large asteroid or comet could have caused food chains to collapse both on land and at sea by producing dust and particulate aerosols and thus inhibiting photosynthesis.[141] Impacts on sulfur-rich rocks could have emitted sulfur oxides precipitating as poisonous acid rain, contributing further to the collapse of food chains. Such impacts could also have caused megatsunamis and/or global forest fires.
Most paleontologists now agree that an asteroid did hit the Earth about 66 Ma, but there is lingering dispute whether the impact was the sole cause of the Cretaceous–Paleogene extinction event.[142][143] Nonetheless, in October 2019, researchers reported that the Cretaceous Chicxulub asteroid impact that resulted in the extinction of non-avian dinosaurs 66 Ma, also rapidly acidified the oceans, producing ecological collapse and long-lasting effects on the climate, and was a key reason for end-Cretaceous mass extinction.[144][145]
The Permian-Triassic extinction event has also been hypothesised to have been caused by an asteroid impact that formed the Araguainha crater due to the estimated date of the crater's formation overlapping with the end-Permian extinction event.[146][147][148] However, this hypothesis has been widely challenged, with the impact hypothesis being rejected by most researchers.[149][150][151]
According to the Shiva hypothesis, the Earth is subject to increased asteroid impacts about once every 27 million years because of the Sun's passage through the plane of the Milky Way galaxy, thus causing extinction events at 27 million year intervals. Some evidence for this hypothesis has emerged in both marine and non-marine contexts.[152] Alternatively, the Sun's passage through the higher density spiral arms of the galaxy could coincide with mass extinction on Earth, perhaps due to increased impact events.[153] However, a reanalysis of the effects of the Sun's transit through the spiral structure based on maps of the spiral structure of the Milky Way in CO molecular line emission has failed to find a correlation.[154]
A nearby nova, supernova or gamma ray burst
A nearby gamma-ray burst (less than 6000 light-years away) would be powerful enough to destroy the Earth's ozone layer, leaving organisms vulnerable to ultraviolet radiation from the Sun.[155] Gamma ray bursts are fairly rare, occurring only a few times in a given galaxy per million years.[156] It has been suggested that a gamma ray burst caused the End-Ordovician extinction,[157][158] while a supernova has been proposed as the cause of the Hangenberg event.[159]
Global cooling
Sustained and significant global cooling could kill many polar and temperate species and force others to migrate towards the equator; reduce the area available for tropical species; often make the Earth's climate more arid on average, mainly by locking up more of the planet's water in ice and snow. The glaciation cycles of the current ice age are believed to have had only a very mild impact on biodiversity, so the mere existence of a significant cooling is not sufficient on its own to explain a mass extinction.
It has been suggested that global cooling caused or contributed to the End-Ordovician, Permian–Triassic, Late Devonian extinctions, and possibly others. Sustained global cooling is distinguished from the temporary climatic effects of flood basalt events or impacts.
Global warming
This would have the opposite effects: expand the area available for tropical species; kill temperate species or force them to migrate towards the poles; possibly cause severe extinctions of polar species; often make the Earth's climate wetter on average, mainly by melting ice and snow and thus increasing the volume of the water cycle. It might also cause anoxic events in the oceans (see below).
Global warming as a cause of mass extinction is supported by several recent studies.[160]
The most dramatic example of sustained warming is the Paleocene–Eocene Thermal Maximum, which was associated with one of the smaller mass extinctions. It has also been suggested to have caused the Triassic–Jurassic extinction event, during which 20% of all marine families became extinct. Furthermore, the Permian–Triassic extinction event has been suggested to have been caused by warming.[161][162][163]
Clathrate gun hypothesis
Clathrates are composites in which a lattice of one substance forms a cage around another. Methane clathrates (in which water molecules are the cage) form on continental shelves. These clathrates are likely to break up rapidly and release the methane if the temperature rises quickly or the pressure on them drops quickly—for example in response to sudden global warming or a sudden drop in sea level or even earthquakes. Methane is a much more powerful greenhouse gas than carbon dioxide, so a methane eruption ("clathrate gun") could cause rapid global warming or make it much more severe if the eruption was itself caused by global warming.
The most likely signature of such a methane eruption would be a sudden decrease in the ratio of carbon-13 to carbon-12 in sediments, since methane clathrates are low in carbon-13; but the change would have to be very large, as other events can also reduce the percentage of carbon-13.[164]
It has been suggested that "clathrate gun" methane eruptions were involved in the end-Permian extinction ("the Great Dying") and in the Paleocene–Eocene Thermal Maximum, which was associated with one of the smaller mass extinctions.
Anoxic events
Anoxic events are situations in which the middle and even the upper layers of the ocean become deficient or totally lacking in oxygen. Their causes are complex and controversial, but all known instances are associated with severe and sustained global warming, mostly caused by sustained massive volcanism.[165]
It has been suggested that anoxic events caused or contributed to the Ordovician–Silurian,[166][167][168] late Devonian,[169][170][171] Capitanian,[172][173][174] Permian–Triassic,[175][176][177] and Triassic–Jurassic extinctions,[178] as well as a number of lesser extinctions (such as the Ireviken, Lundgreni, Mulde, Lau, Smithian-Spathian, Toarcian, and Cenomanian–Turonian events). On the other hand, there are widespread black shale beds from the mid-Cretaceous that indicate anoxic events but are not associated with mass extinctions.
The bio-availability of essential trace elements (in particular selenium) to potentially lethal lows has been shown to coincide with, and likely have contributed to, at least three mass extinction events in the oceans, that is, at the end of the Ordovician, during the Middle and Late Devonian, and at the end of the Triassic. During periods of low oxygen concentrations very soluble selenate (Se6+) is converted into much less soluble selenide (Se2-), elemental Se and organo-selenium complexes. Bio-availability of selenium during these extinction events dropped to about 1% of the current oceanic concentration, a level that has been proven lethal to many extant organisms.[179]
British oceanologist and atmospheric scientist, Andrew Watson, explained that, while the Holocene epoch exhibits many processes reminiscent of those that have contributed to past anoxic events, full-scale ocean anoxia would take "thousands of years to develop".[180]
Hydrogen sulfide emissions from the seas
Kump, Pavlov and Arthur (2005) have proposed that during the Permian–Triassic extinction event the warming also upset the oceanic balance between photosynthesising plankton and deep-water sulfate-reducing bacteria, causing massive emissions of hydrogen sulfide, which poisoned life on both land and sea and severely weakened the ozone layer, exposing much of the life that still remained to fatal levels of UV radiation.[181][182][72]
Oceanic overturn
Oceanic overturn is a disruption of thermo-haline circulation that lets surface water (which is more saline than deep water because of evaporation) sink straight down, bringing anoxic deep water to the surface and therefore killing most of the oxygen-breathing organisms that inhabit the surface and middle depths. It may occur either at the beginning or the end of a glaciation, although an overturn at the start of a glaciation is more dangerous because the preceding warm period will have created a larger volume of anoxic water.[183]
Unlike other oceanic catastrophes such as regressions (sea-level falls) and anoxic events, overturns do not leave easily identified "signatures" in rocks and are theoretical consequences of researchers' conclusions about other climatic and marine events.
It has been suggested that oceanic overturn caused or contributed to the late Devonian and Permian–Triassic extinctions.
Geomagnetic reversal
One theory is that periods of increased geomagnetic reversals will weaken Earth's magnetic field long enough to expose the atmosphere to the solar winds, causing oxygen ions to escape the atmosphere in a rate increased by 3–4 orders, resulting in a disastrous decrease in oxygen.[184]
Plate tectonics
Movement of the continents into some configurations can cause or contribute to extinctions in several ways: by initiating or ending ice ages; by changing ocean and wind currents and thus altering climate; by opening seaways or land bridges that expose previously isolated species to competition for which they are poorly adapted (for example, the extinction of most of South America's native ungulates and all of its large metatherians after the creation of a land bridge between North and South America). Occasionally continental drift creates a super-continent that includes the vast majority of Earth's land area, which in addition to the effects listed above is likely to reduce the total area of continental shelf (the most species-rich part of the ocean) and produce a vast, arid continental interior that may have extreme seasonal variations.
Another theory is that the creation of the super-continent Pangaea contributed to the End-Permian mass extinction. Pangaea was almost fully formed at the transition from mid-Permian to late-Permian, and the "Marine genus diversity" diagram at the top of this article shows a level of extinction starting at that time, which might have qualified for inclusion in the "Big Five" if it were not overshadowed by the "Great Dying" at the end of the Permian.[185]
Other hypotheses
.jpg.webp)
Many other hypotheses have been proposed, such as the spread of a new disease, or simple out-competition following an especially successful biological innovation. But all have been rejected, usually for one of the following reasons: they require events or processes for which there is no evidence; they assume mechanisms that are contrary to the available evidence; they are based on other theories that have been rejected or superseded.

Scientists have been concerned that human activities could cause more plants and animals to become extinct than any point in the past. Along with human-made changes in climate (see above), some of these extinctions could be caused by overhunting, overfishing, invasive species, or habitat loss. A study published in May 2017 in Proceedings of the National Academy of Sciences argued that a "biological annihilation" akin to a sixth mass extinction event is underway as a result of anthropogenic causes, such as over-population and over-consumption. The study suggested that as much as 50% of the number of animal individuals that once lived on Earth were already extinct, threatening the basis for human existence too.[187][30]
Future biosphere extinction/sterilization
The eventual warming and expanding of the Sun, combined with the eventual decline of atmospheric carbon dioxide, could actually cause an even greater mass extinction, having the potential to wipe out even microbes (in other words, the Earth would be completely sterilized): rising global temperatures caused by the expanding Sun would gradually increase the rate of weathering, which would in turn remove more and more CO2 from the atmosphere. When CO2 levels get too low (perhaps at 50 ppm), most plant life will die out, although simpler plants like grasses and mosses can survive much longer, until CO2 levels drop to 10 ppm.[188][189]
With all photosynthetic organisms gone, atmospheric oxygen can no longer be replenished, and it is eventually removed by chemical reactions in the atmosphere, perhaps from volcanic eruptions. Eventually the loss of oxygen will cause all remaining aerobic life to die out via asphyxiation, leaving behind only simple anaerobic prokaryotes. When the Sun becomes 10% brighter in about a billion years,[188] Earth will suffer a moist greenhouse effect resulting in its oceans boiling away, while the Earth's liquid outer core cools due to the inner core's expansion and causes the Earth's magnetic field to shut down. In the absence of a magnetic field, charged particles from the Sun will deplete the atmosphere and further increase the Earth's temperature to an average of around 420 K (147 °C, 296 °F) in 2.8 billion years, causing the last remaining life on Earth to die out. This is the most extreme instance of a climate-caused extinction event. Since this will only happen late in the Sun's life, it would represent the final mass extinction in Earth's history (albeit a very long extinction event).[188][189]
Effects and recovery
The effects of mass extinction events varied widely. After a major extinction event, usually only weedy species survive due to their ability to live in diverse habitats.[190] Later, species diversify and occupy empty niches. Generally, it takes millions of years for biodiversity to recover after extinction events.[191] In the most severe mass extinctions it may take 15 to 30 million years.[190]
The worst Phanerozoic event, the Permian–Triassic extinction, devastated life on Earth, killing over 90% of species. Life seemed to recover quickly after the P-T extinction, but this was mostly in the form of disaster taxa, such as the hardy Lystrosaurus. The most recent research indicates that the specialized animals that formed complex ecosystems, with high biodiversity, complex food webs and a variety of niches, took much longer to recover. It is thought that this long recovery was due to successive waves of extinction that inhibited recovery, as well as prolonged environmental stress that continued into the Early Triassic. Recent research indicates that recovery did not begin until the start of the mid-Triassic, four to six million years after the extinction;[192] and some writers estimate that the recovery was not complete until 30 million years after the P-T extinction, that is, in the late Triassic.[193] Subsequent to the P-T extinction, there was an increase in provincialization, with species occupying smaller ranges – perhaps removing incumbents from niches and setting the stage for an eventual rediversification.[194]
The effects of mass extinctions on plants are somewhat harder to quantify, given the biases inherent in the plant fossil record. Some mass extinctions (such as the end-Permian) were equally catastrophic for plants, whereas others, such as the end-Devonian, did not affect the flora.[195]
See also
- Bioevent
- Elvis taxon
- Endangered species
- Geologic time scale
- Global catastrophic risk
- Holocene extinction
- Human extinction
- Kačák Event
- Lazarus taxon
- List of impact craters on Earth
- List of largest volcanic eruptions
- List of possible impact structures on Earth
- Medea hypothesis
- Rare species
- Signor–Lipps effect
- Snowball Earth
- Speculative evolution
- The Sixth Extinction: An Unnatural History (nonfiction book)
- Timeline of extinctions in the Holocene
- Quaternary extinction event
Footnotes
- Biodiversity is declining faster than at any time in human history. Current extinction rates, for example, are around 100~1,000 times higher than the baseline rate, and they are increasing.[27]
- "The ongoing sixth mass extinction may be the most serious environmental threat to the persistence of civilization, because it is irreversible. Thousands of populations of critically endangered vertebrate animal species have been lost in a century, indicating that the sixth mass extinction is human caused and accelerating. The acceleration of the extinction crisis is certain because of the still fast growth in human numbers and consumption rates."[32]
- Dissolved oxygen became more widespread and penetrated to greater depths; the development of life on land reduced the run-off of nutrients and hence the risk of eutrophication and anoxic events; and marine ecosystems became more diversified so that food chains were less likely to be disrupted.
- The earliest known flood basalt event is the one that produced the Siberian Traps and is associated with the end-Permian extinction.
- Some of the extinctions associated with flood basalts and sea-level falls were significantly smaller than the "major" extinctions, but still much greater than the background extinction level.
References
- Sudakow, Ivan; Myers, Corinne; Petrovskii, Sergei; Sumrall, Colin D.; Witts, James (July 2022). "Knowledge gaps and missing links in understanding mass extinctions: Can mathematical modeling help?". Physics of Life Reviews. 41: 22–57. Bibcode:2022PhLRv..41...22S. doi:10.1016/j.plrev.2022.04.001. PMID 35523056. S2CID 248215038. Retrieved 4 November 2022.
- Raup DM, Sepkoski JJ (March 1982). "Mass extinctions in the marine fossil record". Science. 215 (4539): 1501–1503. Bibcode:1982Sci...215.1501R. doi:10.1126/science.215.4539.1501. PMID 17788674. S2CID 43002817.
- Alroy J (August 2008). "Colloquium paper: dynamics of origination and extinction in the marine fossil record". Proceedings of the National Academy of Sciences of the United States of America. 105 (Supplement 1): 11536–11542. Bibcode:2008PNAS..10511536A. doi:10.1073/pnas.0802597105. PMC 2556405. PMID 18695240.
- Gould SJ (October 1994). "The Evolution of Life on Earth". Scientific American. Vol. 271, no. 4. pp. 84–91. Bibcode:1994SciAm.271d..84G. doi:10.1038/scientificamerican1094-84. PMID 7939569.
- PNAS:Environmental drivers of the first major animal extinction across the Ediacaran White Sea-Nama transition.
- "extinction". Math.ucr.edu. Retrieved 9 November 2008.
- Hall S (10 June 2020). "Familiar culprit may have caused mysterious mass extinction – A planet heated by giant volcanic eruptions drove the earliest known wipeout of life on Earth". The New York Times. Retrieved 15 June 2020.
- Bond DP, Grasby SE (18 May 2020). "Late Ordovician mass extinction caused by volcanism, warming, and anoxia, not cooling and glaciation". Geology. 48 (8): 777–781. Bibcode:2020Geo....48..777B. doi:10.1130/G47377.1. S2CID 234740291.
- Harper DA, Hammarlund EU, Rasmussen CM (May 2014). "End Ordovician extinctions: A coincidence of causes". Gondwana Research. 25 (4): 1294–1307. Bibcode:2014GondR..25.1294H. doi:10.1016/j.gr.2012.12.021.
- Longman J, Mills BJ, Manners HR, Gernon TM, Palmer MR (December 2021). "Late Ordovician climate change and extinctions driven by elevated volcanic nutrient supply" (PDF). Nature Geoscience. 14 (12): 924–929. Bibcode:2021NatGe..14..924L. doi:10.1038/s41561-021-00855-5. S2CID 244803446.
- Briggs D, Crowther PR (2008). Palaeobiology. Vol. II. John Wiley & Sons. p. 223. ISBN 978-0-470-99928-8 – via Google Books.
- McGhee Jr GR, Clapham ME, Sheehan PM, Bottjer DJ, Droser ML (January 2013). "A new ecological-severity ranking of major Phanerozoic biodiversity crises". Palaeogeography, Palaeoclimatology, Palaeoecology. 370: 260–270. Bibcode:2013PPP...370..260M. doi:10.1016/j.palaeo.2012.12.019. ISSN 0031-0182.
- St Fleur N (16 February 2017). "After Earth's worst mass extinction, life rebounded rapidly, fossils suggest". The New York Times. Archived from the original on 1 January 2022. Retrieved 17 February 2017.
- Stanley SM (October 2016). "Estimates of the magnitudes of major marine mass extinctions in earth history". Proceedings of the National Academy of Sciences. 113 (42): E6325–E6334. Bibcode:2016PNAS..113E6325S. doi:10.1073/pnas.1613094113. ISSN 0027-8424. PMC 5081622. PMID 27698119.
- Erwin, Douglas H. (20 January 1994). "The Permo-Triassic extinction". Nature. 367 (6460): 231. Bibcode:1994Natur.367..231E. doi:10.1038/367231a0. S2CID 4328753.
- Labandeira CC, Sepkoski JJ (July 1993). "Insect diversity in the fossil record". Science. 261 (5119): 310–315. Bibcode:1993Sci...261..310L. CiteSeerX 10.1.1.496.1576. doi:10.1126/science.11536548. hdl:10088/6563. PMID 11536548.
- McElwain JC, Punyasena SW (October 2007). "Mass extinction events and the plant fossil record". Trends in Ecology & Evolution. 22 (10): 548–557. doi:10.1016/j.tree.2007.09.003. PMID 17919771.
- Sahney S, Benton MJ (April 2008). "Recovery from the most profound mass extinction of all time". Proceedings. Biological Sciences. 275 (1636): 759–765. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
- Macleod N, Rawson PF, Forey P, Banner F, Boudagher-Fadel M, Bown P, et al. (April 1997). "The Cretaceous-Tertiary biotic transition". Journal of the Geological Society. 154 (2): 265–92. Bibcode:1997JGSoc.154..265M. doi:10.1144/gsjgs.154.2.0265. S2CID 129654916.
- Fastovsky DE, Sheehan PM (2005). "The extinction of the dinosaurs in North America". GSA Today. 15 (3): 4–10. doi:10.1130/1052-5173(2005)15<4:TEOTDI>2.0.CO;2.
- McGhee GR, Sheehan PM, Bottjer DJ, Droser ML (2011). "Ecological ranking of Phanerozoic biodiversity crises: The Serpukhovian (early Carboniferous) crisis had a greater ecological impact than the end-Ordovician". Geology. 40 (2): 147–50. Bibcode:2012Geo....40..147M. doi:10.1130/G32679.1.
- Sole RV, Newman M (2003). "Extinctions and biodiversity in the fossil record". In Mooney HA, Canadell JG (eds.). Encyclopedia of Global Environmental Change, Volume 2, The Earth System: Biological and Ecological Dimensions of Global Environmental Change. Wiley. pp. 297–391. ISBN 978-0-470-85361-0.
- Smith AB, McGowan AJ (December 2005). "Cyclicity in the fossil record mirrors rock outcrop area". Biology Letters. 1 (4): 443–445. doi:10.1098/rsbl.2005.0345. PMC 1626379. PMID 17148228.
- Smith AB, McGowan AJ (2007). "The shape of the Phanerozoic marine palaeodiversity curve: How much can be predicted from the sedimentary rock record of Western Europe?". Palaeontology. 50 (4): 765–74. Bibcode:2007Palgy..50..765S. doi:10.1111/j.1475-4983.2007.00693.x. S2CID 55728929.
- McCallum ML (27 May 2015). "Vertebrate biodiversity losses point to a sixth mass extinction". Biodiversity and Conservation. 24 (10): 2497–2519. doi:10.1007/s10531-015-0940-6. S2CID 16845698.
- Pimm SL, Jenkins CN, Abell R, Brooks TM, Gittleman JL, Joppa LN, et al. (May 2014). "The biodiversity of species and their rates of extinction, distribution, and protection". Science. 344 (6187): 1246752. doi:10.1126/science.1246752. PMID 24876501. S2CID 206552746.
- Dasgupta P (2021). "The Economics of Biodiversity" (PDF). The Dasgupta Review Headline Messages. UK government. p. 1. Retrieved 9 January 2022.
- MacDonald J (3 July 2015). "It's official: A global mass extinction is under way". JSTOR Daily.
- Grennan M (24 June 2015). "We're entering a sixth mass extinction, and it's our fault". Popular Science.
- Sutter JD (11 July 2017). "Sixth mass extinction: The era of 'biological annihilation'". CNN. Retrieved 17 July 2017.
- Cowie RH, Bouchet P, Fontaine B (April 2022) [10 January 2022]. "The Sixth Mass Extinction: fact, fiction or speculation?". Biological Reviews of the Cambridge Philosophical Society (online preprint). 97 (2): 640–663. doi:10.1111/brv.12816. PMC 9786292. PMID 35014169.
- Ceballos G, Ehrlich PR, Raven PH (June 2020). "Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction". Proceedings of the National Academy of Sciences of the United States of America. 117 (24): 13596–13602. Bibcode:2020PNAS..11713596C. doi:10.1073/pnas.1922686117. PMC 7306750. PMID 32482862.
- Brondizio ES, Settele J, Díaz S, Ngo HT, et al. (Intergovernmental Science-Policy Platform On Biodiversity And Ecosystem Services) (25 November 2019). Summary for policymakers of the global assessment report on biodiversity and ecosystem services. Intergovernmental Science-Policy Platform on Biodiversity Ecosystem Services. IPBES plenary seventh session. doi:10.5281/zenodo.3553579. ISBN 978-3-947851-13-3.
- Watts J (6 May 2019). "Human society under urgent threat from loss of Earth's natural life". The Guardian. Retrieved 10 May 2019.
- Plumer B (6 May 2019). "Humans are speeding extinction and altering the natural world at an 'unprecedented' pace". The New York Times. Archived from the original on 1 January 2022. Retrieved 10 May 2019.
- "Nature's dangerous decline 'unprecedented'; species extinction rates 'accelerating'". Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (Press release). 6 May 2019. Retrieved 10 May 2019.
- "Looming mass extinction could be biggest 'since the dinosaurs,' says WWF". Deutsche Welle. Germany. 29 December 2021. Retrieved 3 January 2022.
- Rozsa, Matthew (19 September 2023). "Experts warn of a "biological holocaust" as human-caused extinction "mutilates" the tree of life". Salon.com. Retrieved 21 September 2023.
- Ceballos, Gerardo; Ehrlich, Paul R. (2023). "Mutilation of the tree of life via mass extinction of animal genera". Proceedings of the National Academy of Sciences of the United States of America. 120 (39): e2306987120. doi:10.1073/pnas.2306987120.
- Greenfield, Patrick (19 September 2023). "'Mutilating the tree of life': Wildlife loss accelerating, scientists warn". The Guardian. Retrieved 21 September 2023.
- Sepkoski, J.J. Jr. (1982). A compendium of fossil marine families (PDF) (Report). Milwaukee Public Museum Contributions in Biology and Geology. Vol. 51. pp. 1–125.
- Sepkoski Jr JJ (1992). A compendium of fossil marine animal families (PDF) (Report). Milwaukee Public Museum Contributions in Biology and Geology. Vol. 83 (2nd ed.). pp. 1–156. PMID 11542296.
- Sepkoski Jr JJ (2002). Jablonski D, Foote M (eds.). "A Compendium of Fossil Marine Animal Genera". Bulletins of American Paleontology. 363: 1–560.
- Sepkoski JJ (1996). "Patterns of Phanerozoic Extinction: A Perspective from Global Data Bases". In Walliser OH (ed.). Global Events and Event Stratigraphy in the Phanerozoic. Berlin & Heidelberg, DE: Springer Berlin Heidelberg. pp. 35–51. doi:10.1007/978-3-642-79634-0_4. ISBN 978-3-642-79636-4. Retrieved 14 August 2022.
- Bambach RK (May 2006). "Phanerozoic Biodiversity Mass Extinctions". Annual Review of Earth and Planetary Sciences. 34 (1): 127–155. Bibcode:2006AREPS..34..127B. doi:10.1146/annurev.earth.33.092203.122654. ISSN 0084-6597.
- Alvarez LW, Alvarez W, Asaro F, Michel HV (June 1980). "Extraterrestrial cause for the cretaceous-tertiary extinction". Science. 208 (4448): 1095–1108. Bibcode:1980Sci...208.1095A. CiteSeerX 10.1.1.126.8496. doi:10.1126/science.208.4448.1095. PMID 17783054. S2CID 16017767.
- Sepkoski, J.J. Jr. (1981). "A factor analytic description of the Phanerozoic marine fossil record" (PDF). Paleobiology. 7 (1): 36–53. Bibcode:1981Pbio....7...36S. doi:10.1017/S0094837300003778. ISSN 0094-8373. S2CID 133114885.
- Sepkoski JJ, Bambach RK, Raup DM, Valentine JW (1981). "Phanerozoic marine diversity and the fossil record" (PDF). Nature. 293 (5832): 435–437. Bibcode:1981Natur.293..435S. doi:10.1038/293435a0. ISSN 1476-4687. S2CID 4282371.
- Sepkoski JJ (1 January 1982). "Mass extinctions in the Phanerozoic oceans: A review". Geological Implications of Impacts of Large Asteroids and Comets on the Earth. Geological Society of America Special Papers. Vol. 190. Geological Society of America. pp. 283–290. doi:10.1130/SPE190-p283. ISBN 0-8137-2190-3. Special Paper 190.
- Sepkoski JJ (1984). "A kinetic model of Phanerozoic taxonomic diversity. III. Post-Paleozoic families and mass extinctions". Paleobiology. 10 (2): 246–267. Bibcode:1984Pbio...10..246S. doi:10.1017/S0094837300008186. ISSN 0094-8373. S2CID 85595559.
- Sepkoski JJ (1986). "Phanerozoic overview of mass extinction". In Raup DM, Jablonski D (eds.). Patterns and Processes in the History of Life. Dahlem Workshop Reports. Berlin & Heidelberg, DE: Springer Berlin Heidelberg. pp. 277–295. doi:10.1007/978-3-642-70831-2_15. ISBN 978-3-642-70833-6. Retrieved 14 August 2022.
- Sepkoski JJ (1989). "Periodicity in extinction and the problem of catastrophism in the history of life". Journal of the Geological Society. 146 (1): 7–19. Bibcode:1989JGSoc.146....7S. doi:10.1144/gsjgs.146.1.0007. PMID 11539792. S2CID 45567004.
- Raup DM, Sepkoski JJ (February 1984). "Periodicity of extinctions in the geologic past". Proceedings of the National Academy of Sciences of the United States of America. 81 (3): 801–805. Bibcode:1984PNAS...81..801R. doi:10.1073/pnas.81.3.801. PMC 344925. PMID 6583680.
- Sepkoski JJ (1993). "Ten years in the library: New data confirm paleontological patterns". Paleobiology. 19 (1): 43–51. Bibcode:1993Pbio...19...43S. doi:10.1017/S0094837300012306. PMID 11538041. S2CID 44295283.
- Jablonski D (August 1991). "Extinctions: A paleontological perspective". Science. 253 (5021): 754–757. Bibcode:1991Sci...253..754J. doi:10.1126/science.253.5021.754. PMID 17835491.
- Benton MJ (April 1995). "Diversification and extinction in the history of life" (PDF). Science. 268 (5207): 52–58. Bibcode:1995Sci...268...52B. doi:10.1126/science.7701342. PMID 7701342.
- Walliser OH, ed. (1996). Global Events and Event Stratigraphy in the Phanerozoic: Results of the International Interdisciplinary Cooperation in the IGCP-Project 216 "Global Biological Events in Earth History". Berlin, Heidelberg: Springer Berlin Heidelberg. doi:10.1007/978-3-642-79634-0. ISBN 978-3-642-79636-4.
- Hallam A, Wignall PB (1997). Mass Extinctions and Their Aftermath. Oxford: Oxford University Press.
- Barnes CR, Hallam A, Kaljo D, Kauffman EG, Walliser OH (1996). "Global Event Stratigraphy". Global Events and Event Stratigraphy in the Phanerozoic. Berlin & Heidelberg, DE: Springer Berlin Heidelberg. pp. 319–333. doi:10.1007/978-3-642-79634-0_16. ISBN 978-3-642-79636-4.
- Foote M (2000). "Origination and extinction components of taxonomic diversity: General problems". Paleobiology. 26 (S4): 74–102. Bibcode:2000Pbio...26S..74F. doi:10.1017/S0094837300026890. ISSN 0094-8373. S2CID 53341052.
- Bambach RK, Knoll AH, Wang SC (2004). "Origination, extinction, and mass depletions of marine diversity". Paleobiology. 30 (4): 522–542. doi:10.1666/0094-8373(2004)030<0522:OEAMDO>2.0.CO;2. ISSN 0094-8373. S2CID 17279135.
- Foote M (2005). "Pulsed origination and extinction in the marine realm" (PDF). Paleobiology. 31 (1): 6–20. doi:10.1666/0094-8373(2005)031<0006:POAEIT>2.0.CO;2. S2CID 53469954.
- Stanley SM (2007). "Memoir 4: An Analysis of the History of Marine Animal Diversity". Paleobiology. 33 (S4): 1–55. Bibcode:2007Pbio...33Q...1S. doi:10.1017/S0094837300019217. ISSN 0094-8373. S2CID 90130435.
- Signor III, P. W. and Lipps, J. H. (1982) "Sampling bias, gradual extinction patterns, and catastrophes in the fossil record", in Geological implications of impacts of large asteroids and comets on the Earth (ed. L. T. Silver and P. H. Schultz), Geological Society of America Special Publication, vol. 190, pp. 291–296.
- Foote M (2007). "Extinction and quiescence in marine animal genera". Paleobiology. 33 (2): 261–272. doi:10.1666/06068.1. ISSN 0094-8373. S2CID 53402257.
- Alroy J (2010). "Fair Sampling of Taxonomic Richness and Unbiased Estimation of Origination and Extinction Rates". The Paleontological Society Papers. 16: 55–80. doi:10.1017/s1089332600001819. ISSN 1089-3326.
- Alroy J (2014). "Accurate and precise estimates of origination and extinction rates". Paleobiology. 40 (3): 374–397. doi:10.1666/13036. ISSN 0094-8373. S2CID 53125415.
- Alroy J (2015). "A more precise speciation and extinction rate estimator". Paleobiology. 41 (4): 633–639. Bibcode:2015Pbio...41..633A. doi:10.1017/pab.2015.26. ISSN 0094-8373. S2CID 85842940.
- Nee S (August 2004). "Extinction, slime, and bottoms". PLOS Biology. 2 (8): E272. doi:10.1371/journal.pbio.0020272. PMC 509315. PMID 15314670.
- Butterfield NJ (2007). "Macroevolution and macroecology through deep time" (PDF). Palaeontology. 50 (1): 41–55. Bibcode:2007Palgy..50...41B. doi:10.1111/j.1475-4983.2006.00613.x. S2CID 59436643. Archived from the original (PDF) on 21 July 2022. Retrieved 6 October 2019.
- Plait P (28 July 2014). "Poisoned Planet". Slate. Retrieved 8 July 2019.
- Ward PD (October 2006). "Impact from the deep". Scientific American. Vol. 295, no. 4. pp. 64–71. Bibcode:2006SciAm.295d..64W. doi:10.1038/scientificamerican1006-64. PMID 16989482.
-
Kluger J (25 July 2014). "The Sixth Great Extinction is underway – and we're to blame". Time. Retrieved 14 December 2016.
- Benton MJ (2004). "6. Reptiles Of the Triassic". Vertebrate Palaeontology. Blackwell. ISBN 978-0-04-566002-5.
- van Valkenburgh B (1999). "Major patterns in the history of carnivorous mammals". Annual Review of Earth and Planetary Sciences. 27: 463–93. Bibcode:1999AREPS..27..463V. doi:10.1146/annurev.earth.27.1.463.
- Jablonski D (June 2002). "Survival without recovery after mass extinctions". Proceedings of the National Academy of Sciences of the United States of America. 99 (12): 8139–8144. Bibcode:2002PNAS...99.8139J. doi:10.1073/pnas.102163299. PMC 123034. PMID 12060760.
- Budd GE, Mann RP (November 2018). "History is written by the victors: The effect of the push of the past on the fossil record". Evolution; International Journal of Organic Evolution. 72 (11): 2276–2291. doi:10.1111/evo.13593. PMC 6282550. PMID 30257040.
- Hallam A, Wignall PB (2002). Mass Extinctions and their Aftermath. New York, NY: Oxford University Press.
- Beardsley T (1988). "Star-struck?". Scientific American. Vol. 258, no. 4. pp. 37–40. Bibcode:1988SciAm.258d..37B. doi:10.1038/scientificamerican0488-37b.
- Different cycle lengths have been proposed; e.g. by Rohde RA, Muller RA (March 2005). "Cycles in fossil diversity". Nature. 434 (7030): 208–210. Bibcode:2005Natur.434..208R. doi:10.1038/nature03339. PMID 15758998. S2CID 32520208.
- Muller RA. "Nemesis". Muller.lbl.gov. Lawrence Berkeley Laboratory. Retrieved 19 May 2007.
- Melott AL, Bambach RK (July 2010). "Nemesis reconsidered". Monthly Notices of the Royal Astronomical Society. 407 (1): L99–L102. arXiv:1007.0437. Bibcode:2010MNRAS.407L..99M. doi:10.1111/j.1745-3933.2010.00913.x. S2CID 7911150. Retrieved 2 July 2010.
- Gillman M, Erenler H (2008). "The galactic cycle of extinction" (PDF). International Journal of Astrobiology. 7 (1): 17–26. Bibcode:2008IJAsB...7...17G. CiteSeerX 10.1.1.384.9224. doi:10.1017/S1473550408004047. ISSN 1475-3006. S2CID 31391193. Retrieved 2 April 2018.
- Bailer-Jones CA (July 2009). "The evidence for and against astronomical impacts on climate change and mass extinctions: A review". International Journal of Astrobiology. 8 (3): 213–219. arXiv:0905.3919. Bibcode:2009IJAsB...8..213B. doi:10.1017/S147355040999005X. ISSN 1475-3006. S2CID 2028999.
- Overholt AC, Melott AL, Pohl M (2009). "Testing the link between terrestrial climate change and galactic spiral arm transit". The Astrophysical Journal. 705 (2): L101–03. arXiv:0906.2777. Bibcode:2009ApJ...705L.101O. doi:10.1088/0004-637X/705/2/L101. S2CID 734824.
- Erlykin AD, Harper DA, Sloan T, Wolfendale AW (2017). Smith A (ed.). "Mass extinctions over the last 500 myr: an astronomical cause?". Palaeontology. 60 (2): 159–167. Bibcode:2017Palgy..60..159E. doi:10.1111/pala.12283. S2CID 133407217.
- Melott AL, Bambach RK (2011). "A[n] ubiquitous ~62 Myr periodic fluctuation superimposed on general trends in fossil biodiversity. I. Documentation". Paleobiology. 37: 92–112. arXiv:1005.4393. doi:10.1666/09054.1. S2CID 1905891.
- Melott AL, Bambach RK, Petersen KD, McArthur JM, et al. (2012). "A ~60 Myr periodicity is common to marine-87Sr/86Sr, fossil biodiversity, and large-scale sedimentation: what does the periodicity reflect?". Journal of Geology. 120 (2): 217–226. arXiv:1206.1804. Bibcode:2012JG....120..217M. doi:10.1086/663877. S2CID 18027758.
- Müller RD, Dutkiewicz A (February 2018). "Oceanic crustal carbon cycle drives 26-million-year atmospheric carbon dioxide periodicities". Science Advances. 4 (2): eaaq0500. Bibcode:2018SciA....4..500M. doi:10.1126/sciadv.aaq0500. PMC 5812735. PMID 29457135.
- Arens NC, West ID (2008). "Press-pulse: a general theory of mass extinction?" (PDF). Paleobiology. 34 (4): 456–471. Bibcode:2008Pbio...34..456A. doi:10.1666/07034.1. S2CID 56118514.
- Wang SC, Bush AM (2008). "Adjusting global extinction rates to account for taxonomic susceptibility". Paleobiology. 34 (4): 434–55. doi:10.1666/07060.1. S2CID 16260671.
- Budd GE (February 2003). "The Cambrian fossil record and the origin of the phyla". Integrative and Comparative Biology. 43 (1): 157–165. doi:10.1093/icb/43.1.157. PMID 21680420.
- Martin RE (1995). "Cyclic and secular variation in microfossil biomineralization: Clues to the biogeochemical evolution of Phanerozoic oceans". Global and Planetary Change. 11 (1): 1–23. Bibcode:1995GPC....11....1M. doi:10.1016/0921-8181(94)00011-2.
- Martin RE (1996). "Secular increase in nutrient levels through the Phanerozoic: Implications for productivity, biomass, and diversity of the marine biosphere". PALAIOS. 11 (3): 209–219. Bibcode:1996Palai..11..209M. doi:10.2307/3515230. JSTOR 3515230.
- Marshall CR, Ward PD (November 1996). "Sudden and Gradual Molluscan Extinctions in the Latest Cretaceous of Western European Tethys". Science. 274 (5291): 1360–1363. Bibcode:1996Sci...274.1360M. doi:10.1126/science.274.5291.1360. PMID 8910273. S2CID 1837900.
- Arens NC, West ID (2006). Press/pulse: A general theory of mass extinction?. Geological Society of America. Archived from the original on 18 January 2017.
- MacLeod N (6 January 2001). "Extinction!". firstscience.com.
- Courtillot V, Jaeger JJ, Yang Z, Feraud G, Hofmann C (1996). "The influence of continental flood basalts on mass extinctions: Where do we stand?". The Cretaceous-Tertiary Event and other Catastrophes in Earth History. doi:10.1130/0-8137-2307-8.513. ISBN 9780813723075.
- Hallam A (1992). Phanerozoic sea-level changes. New York, NY: Columbia University Press. ISBN 978-0-231-07424-7.
- Grieve RA, Pesonen LJ (December 1992). "The Terrestrial Impact Cratering Record". Tectonophysics. 216 (1–2): 1–30. Bibcode:1992Tectp.216....1G. doi:10.1016/0040-1951(92)90152-V.
- Wignall PB (2001). "Large igneous provinces and mass extinctions". Earth-Science Reviews. 53 (1–2): 1–33. Bibcode:2001ESRv...53....1W. doi:10.1016/S0012-8252(00)00037-4.
- Brannen P (2017). The Ends of the World: Volcanic Apocalypses, Lethal Oceans, and Our Quest to Understand Earth's Past Mass Extinctions. Harper Collins. p. 336. ISBN 978-0-06-236480-7.
- Morrow JR, Sandberg CA (2005). Revised Dating Of Alamo And Some Other Late Devonian Impacts In Relation To Resulting Mass Extinction (PDF). 68th Annual Meteoritical Society Meeting.
- Courtillot VE (1990). "A volcanic eruption". Scientific American. Vol. 263, no. 4. pp. 85–93. Bibcode:1990SciAm.263d..85C. doi:10.1038/scientificamerican1090-85. JSTOR 24997065. PMID 11536474.
- Rampino, Michael R. (13 April 2010). "Mass extinctions of life and catastrophic flood basalt volcanism". Proceedings of the National Academy of Sciences of the United States of America. 107 (15): 6555–6556. Bibcode:2010PNAS..107.6555R. doi:10.1073/pnas.1002478107. PMC 2872464. PMID 20360556.
- Bryan, Scott E.; Peate, Ingrid Ukstins; Peate, David W.; Self, Stephen; Jerram, Dougal A.; Mawby, Michael R.; Marsh, J. S. (Goonie); Miller, Jodie A. (October 2010). "The largest volcanic eruptions on Earth". Earth-Science Reviews. 102 (3–4): 207–229. Bibcode:2010ESRv..102..207B. doi:10.1016/j.earscirev.2010.07.001. Retrieved 11 January 2023.
- Ricci, J.; et al. (2013). "New 40Ar/39Ar and K–Ar ages of the Viluy traps (Eastern Siberia): Further evidence for a relationship with the Frasnian–Famennian mass extinction". Palaeogeography, Palaeoclimatology, Palaeoecology. 386: 531–540. doi:10.1016/j.palaeo.2013.06.020.
- Bond, D. P. G.; Wignall, P. B. (2014). "Large igneous provinces and mass extinctions: An update". GSA Special Papers. 505: 29–55. doi:10.1130/2014.2505(02). ISBN 9780813725055. Retrieved 23 December 2022.
- Kaiho, Kunio; Miura, Mami; Tezuka, Mio; Hayashi, Naohiro; Jones, David S.; Oikawa, Kazuma; Casier, Jean-Georges; Fujibayashi, Megumu; Chen, Zhong-Qiang (April 2021). "Coronene, mercury, and biomarker data support a link between extinction magnitude and volcanic intensity in the Late Devonian". Global and Planetary Change. 199: 103452. Bibcode:2021GPC...19903452K. doi:10.1016/j.gloplacha.2021.103452. S2CID 234364043. Retrieved 11 January 2023.
- Jerram, Dougal A.; Widdowson, Mike; Wignall, Paul B.; Sun, Yadong; Lai, Xulong; Bond, David P. G.; Torsvik, Trond H. (1 January 2016). "Submarine palaeoenvironments during Emeishan flood basalt volcanism, SW China: Implications for plume–lithosphere interaction during the Capitanian, Middle Permian ('end Guadalupian') extinction event". Palaeogeography, Palaeoclimatology, Palaeoecology. 441: 65–73. Bibcode:2016PPP...441...65J. doi:10.1016/j.palaeo.2015.06.009. Retrieved 11 January 2023.
- Retallack, Gregory J.; Jahren, A. Hope (1 October 2007). "Methane Release from Igneous Intrusion of Coal during Late Permian Extinction Events". The Journal of Geology. 116 (1): 1–20. doi:10.1086/524120. S2CID 46914712. Retrieved 11 January 2023.
- Sheldon, Nathan D.; Chakrabarti, Ramananda; Retallack, Gregory J.; Smith, Roger M. H. (20 February 2014). "Contrasting geochemical signatures on land from the Middle and Late Permian extinction events". Sedimentology. 61 (6): 1812–1829. doi:10.1111/sed.12117. hdl:2027.42/108696. S2CID 129862176. Retrieved 11 January 2023.
- Kamo, SL (2003). "Rapid eruption of Siberian flood-volcanic rocks and evidence for coincidence with the Permian–Triassic boundary and mass extinction at 251 Ma". Earth and Planetary Science Letters. 214 (1–2): 75–91. Bibcode:2003E&PSL.214...75K. doi:10.1016/S0012-821X(03)00347-9.
- Jurikova, Hana; Gutjahr, Marcus; Wallmann, Klaus; Flögel, Sascha; Liebetrau, Volker; Posenato, Renato; et al. (November 2020). "Permian–Triassic mass extinction pulses driven by major marine carbon cycle perturbations". Nature Geoscience. 13 (11): 745–750. Bibcode:2020NatGe..13..745J. doi:10.1038/s41561-020-00646-4. ISSN 1752-0908. S2CID 224783993. Retrieved 11 January 2023.
- Burgess, S. D.; Muirhead, J. D.; Bowring, S. A. (31 July 2017). "Initial pulse of Siberian Traps sills as the trigger of the end-Permian mass extinction". Nature Communications. 8 (1): 164. Bibcode:2017NatCo...8..164B. doi:10.1038/s41467-017-00083-9. PMC 5537227. PMID 28761160. S2CID 3312150.
- Paton, M. T.; Ivanov, A. V.; Fiorentini, M. L.; McNaughton, M. J.; Mudrovska, I.; Reznitskii, L. Z.; Demonterova, E. I. (1 September 2010). "Late Permian and Early Triassic magmatic pulses in the Angara–Taseeva syncline, Southern Siberian Traps and their possible influence on the environment". Russian Geology and Geophysics. 51 (9): 1012–1020. Bibcode:2010RuGG...51.1012P. doi:10.1016/j.rgg.2010.08.009. Retrieved 11 January 2023.
- Song, Haijin; Song, Huyue; Tong, Jinnan; Gordon, Gwyneth W.; Wignall, Paul B.; Tian, Li; Zheng, Wang; Algeo, Thomas J.; Liang, Lei; Bai, Ruoyu; Wu, Kui; Anbar, Ariel D. (20 February 2021). "Conodont calcium isotopic evidence for multiple shelf acidification events during the Early Triassic". Chemical Geology. 562: 120038. doi:10.1016/j.chemgeo.2020.120038. S2CID 233915627. Retrieved 11 January 2023.
- Romano, Carlo; Goudemand, Nicolas; Vennemann, Torsten W.; Ware, David; Schneebeli-Hermann, Elke; Hochuli, Peter A.; Brühwiler, Thomas; Brinkmann, Winand; Bucher, Hugo (21 December 2012). "Climatic and biotic upheavals following the end-Permian mass extinction". Nature Geoscience. 6 (1): 57–60. doi:10.1038/ngeo1667. S2CID 129296231.
- Davies, J. H. F. L.; Marzoli, Andrea; Bertrand, H.; Youbi, Nasrrddine; Ernesto, M.; Schaltegger, U. (31 May 2017). "End-Triassic mass extinction started by intrusive CAMP activity". Nature Communications. 8: 15596. Bibcode:2017NatCo...815596D. doi:10.1038/ncomms15596. PMC 5460029. PMID 28561025. S2CID 13323882.
- Blackburn, Terrence J.; Olsen, Paul E.; Bowring, Samuel A.; McLean, Noah M.; Kent, Dennis V; Puffer, John; McHone, Greg; Rasbury, Troy; Et-Touhami7, Mohammed (2013). "Zircon U-Pb Geochronology Links the End-Triassic Extinction with the Central Atlantic Magmatic Province" (PDF). Science. 340 (6135): 941–945. Bibcode:2013Sci...340..941B. CiteSeerX 10.1.1.1019.4042. doi:10.1126/science.1234204. PMID 23519213. S2CID 15895416.
- Capriolo, Manfredo; Mills, Benjamin J. W.; Newton, Robert J.; Corso, Jacobo Dal; Dunhill, Alexander M.; Wignall, Paul B.; Marzoli, Andrea (February 2022). "Anthropogenic-scale CO2 degassing from the Central Atlantic Magmatic Province as a driver of the end-Triassic mass extinction". Global and Planetary Change. 209: 103731. Bibcode:2022GPC...20903731C. doi:10.1016/j.gloplacha.2021.103731. S2CID 245530815. Retrieved 11 January 2023.
- McElwain, Jennifer C.; Wade-Murphy, Jessica; Hesselbo, Stephen P. (26 May 2005). "Changes in carbon dioxide during an oceanic anoxic event linked to intrusion into Gondwana coals". Nature. 435 (7041): 479–482. Bibcode:2005Natur.435..479M. doi:10.1038/nature03618. PMID 15917805. S2CID 4339259. Retrieved 11 January 2023.
- Them, T.R.; Gill, B.C.; Caruthers, A.H.; Gröcke, D.R.; Tulsky, E.T.; Martindale, R.C.; Poulton, T.P.; Smith, P.L. (February 2017). "High-resolution carbon isotope records of the Toarcian Oceanic Anoxic Event (Early Jurassic) from North America and implications for the global drivers of the Toarcian carbon cycle". Earth and Planetary Science Letters. 459: 118–126. Bibcode:2017E&PSL.459..118T. doi:10.1016/j.epsl.2016.11.021.
- Reolid, Matías; Mattioli, Emanuela; Duarte, Luís V.; Ruebsam, Wolfgang (22 September 2021). "The Toarcian Oceanic Anoxic Event: where do we stand?". Geological Society, London, Special Publications. 514 (1): 1–11. Bibcode:2021GSLSP.514....1R. doi:10.1144/SP514-2021-74. ISSN 0305-8719. S2CID 238683028. Retrieved 11 January 2023.
- Kuroda, J; Ogawa, N; Tanimizu, M; Coffin, M; Tokuyama, H; Kitazato, H; Ohkouchi, N (15 April 2007). "Contemporaneous massive subaerial volcanism and late cretaceous Oceanic Anoxic Event 2". Earth and Planetary Science Letters. 256 (1–2): 211–223. Bibcode:2007E&PSL.256..211K. doi:10.1016/j.epsl.2007.01.027. ISSN 0012-821X. S2CID 129546012.
- Flögel, S.; Wallmann, K.; Poulsen, C.J.; Zhou, J.; Oschlies, A.; Voigt, S.; Kuhnt, W. (May 2011). "Simulating the biogeochemical effects of volcanic CO2 degassing on the oxygen-state of the deep ocean during the Cenomanian/Turonian Anoxic Event (OAE2)". Earth and Planetary Science Letters. 305 (3–4): 371–384. Bibcode:2011E&PSL.305..371F. doi:10.1016/j.epsl.2011.03.018. ISSN 0012-821X.
- Ernst, Richard E.; Youbi, Nasrrddine (July 2017). "How Large Igneous Provinces affect global climate, sometimes cause mass extinctions, and represent natural markers in the geological record". Palaeogeography, Palaeoclimatology, Palaeoecology. 478: 30–52. Bibcode:2017PPP...478...30E. doi:10.1016/j.palaeo.2017.03.014.
- Petersen, Sierra V.; Dutton, Andrea; Lohmann, Kyger C. (2016). "End-Cretaceous extinction in Antarctica linked to both Deccan volcanism and meteorite impact via climate change". Nature Communications. 7: 12079. Bibcode:2016NatCo...712079P. doi:10.1038/ncomms12079. PMC 4935969. PMID 27377632.
- Keller, G.; Adatte, T.; Gardin, S.; Bartolini, A.; Bajpai, S. (2008). "Main Deccan volcanism phase ends near the K–T boundary: Evidence from the Krishna-Godavari Basin, SE India". Earth and Planetary Science Letters. 268 (3–4): 293–311. Bibcode:2008E&PSL.268..293K. doi:10.1016/j.epsl.2008.01.015.
- "Causes of the Cretaceous Extinction". park.org/Canada.
- Gutjahr, Marcus; Ridgwell, Andy; Sexton, Philip F.; Anagnostou, Eleni; Pearson, Paul N.; Pälike, Heiko; Norris, Richard D.; Thomas, Ellen; Foster, Gavin L. (August 2017). "Very large release of mostly volcanic carbon during the Palaeocene–Eocene Thermal Maximum". Nature. 548 (7669): 573–577. Bibcode:2017Natur.548..573G. doi:10.1038/nature23646. ISSN 1476-4687. PMC 5582631. PMID 28858305.
- Kender, Sev; Bogus, Kara; Pedersen, Gunver K.; Dybkjær, Karen; Mather, Tamsin A.; Mariani, Erica; Ridgwell, Andy; Riding, James B.; Wagner, Thomas; Hesselbo, Stephen P.; Leng, Melanie J. (31 August 2021). "Paleocene/Eocene carbon feedbacks triggered by volcanic activity". Nature Communications. 12 (1): 5186. Bibcode:2021NatCo..12.5186K. doi:10.1038/s41467-021-25536-0. hdl:10871/126942. ISSN 2041-1723. PMC 8408262. PMID 34465785.
- Jones, Sarah M.; Hoggett, Murray; Greene, Sarah E.; Jones, Tom Dunkley (5 December 2019). "Large Igneous Province thermogenic greenhouse gas flux could have initiated Paleocene-Eocene Thermal Maximum climate change". Nature Communications. 10 (1): 5547. Bibcode:2019NatCo..10.5547J. doi:10.1038/s41467-019-12957-1. PMC 6895149. PMID 31804460.
- Courtillot V (1994). "Mass extinctions in the last 300 million years: one impact and seven flood basalts?". Israel Journal of Earth Sciences. 43: 255–266.
- Courtillot VE, Renne PR (January 2003). "On the ages of flood basalt events". Comptes Rendus Geoscience. 335 (1): 113–140. Bibcode:2003CRGeo.335..113C. doi:10.1016/S1631-0713(03)00006-3.
- Kravchinsky VA (2012). "Paleozoic large igneous provinces of Northern Eurasia: Correlation with mass extinction events" (PDF). Global and Planetary Change. 86: 31–36. Bibcode:2012GPC....86...31K. doi:10.1016/j.gloplacha.2012.01.007.
- Weidlich, O. (2002). "Permian reefs re-examined: extrinsic control mechanisms of gradual and abrupt changes during 40 my of reef evolution". Geobios. 35 (1): 287–294. Bibcode:2002Geobi..35..287W. doi:10.1016/S0016-6995(02)00066-9. Retrieved 8 November 2022.
- Wang, X.-D. & Sugiyama, T. (December 2000). "Diversity and extinction patterns of Permian coral faunas of China". Lethaia. 33 (4): 285–294. doi:10.1080/002411600750053853. Retrieved 8 November 2022.
- Peters SE (July 2008). "Environmental determinants of extinction selectivity in the fossil record" (PDF). Nature. 454 (7204): 626–629. Bibcode:2008Natur.454..626P. doi:10.1038/nature07032. PMID 18552839. S2CID 205213600.
- "Ebb and flow of the sea drives world's big extinction events". Newswise. Madison, WI: University of Wisconsin. 13 June 2008. Retrieved 15 June 2008.
- Alvarez W, Kauffman EG, Surlyk F, Alvarez LW, Asaro F, Michel HV (March 1984). "Impact theory of mass extinctions and the invertebrate fossil record". Science. 223 (4641): 1135–1141. Bibcode:1984Sci...223.1135A. doi:10.1126/science.223.4641.1135. JSTOR 1692570. PMID 17742919. S2CID 24568931.
- Keller G, Abramovich S, Berner Z, Adatte T (1 January 2009). "Biotic effects of the Chicxulub impact, K–T catastrophe and sea level change in Texas". Palaeogeography, Palaeoclimatology, Palaeoecology. 271 (1–2): 52–68. Bibcode:2009PPP...271...52K. doi:10.1016/j.palaeo.2008.09.007.
- Morgan J, Lana C, Kersley A, Coles B, Belcher C, Montanari S, Diaz-Martinez E, Barbosa A, Neumann V (2006). "Analyses of shocked quartz at the global K-P boundary indicate an origin from a single, high-angle, oblique impact at Chicxulub" (PDF). Earth and Planetary Science Letters. 251 (3–4): 264–279. Bibcode:2006E&PSL.251..264M. doi:10.1016/j.epsl.2006.09.009. hdl:10044/1/1208.
- Joel L (21 October 2019). "The dinosaur-killing asteroid acidified the ocean in a flash: The Chicxulub event was as damaging to life in the oceans as it was to creatures on land, a study shows". The New York Times. Archived from the original on 1 January 2022. Retrieved 22 October 2019.
- Henehan MJ, Ridgwell A, Thomas E, Zhang S, Alegret L, Schmidt DN, et al. (November 2019). "Rapid ocean acidification and protracted Earth system recovery followed the end-Cretaceous Chicxulub impact". Proceedings of the National Academy of Sciences of the United States of America. 116 (45): 22500–22504. Bibcode:2019PNAS..11622500H. doi:10.1073/pnas.1905989116. PMC 6842625. PMID 31636204.
- Tohver, Eric; Cawood, P. A.; Riccomini, Claudio; Lana, Cris; Trindade, R. I. F. (1 October 2013). "Shaking a methane fizz: Seismicity from the Araguainha impact event and the Permian–Triassic global carbon isotope record". Palaeogeography, Palaeoclimatology, Palaeoecology. 387: 66–75. Bibcode:2013PPP...387...66T. doi:10.1016/j.palaeo.2013.07.010. Retrieved 12 January 2023.
- Tohver, Eric; Schmieder, Martin; Lana, Cris; Mendes, Pedro S. T.; Jourdan, Fred; Warren, Lucas; Riccomini, Claudio (2 January 2018). "End-Permian impactogenic earthquake and tsunami deposits in the intracratonic Paraná Basin of Brazil". GSA Bulletin. 130 (7–8): 1099–1120. Bibcode:2018GSAB..130.1099T. doi:10.1130/B31626.1. Retrieved 12 January 2023.
- Tohver, Eric; Lana, Cris; Cawood, P.A.; Fletcher, I.R.; Jourdan, F.; Sherlock, S.; et al. (1 June 2012). "Geochronological constraints on the age of a Permo–Triassic impact event: U–Pb and 40Ar / 39Ar results for the 40 km Araguainha structure of central Brazil". Geochimica et Cosmochimica Acta. 86: 214–227. Bibcode:2012GeCoA..86..214T. doi:10.1016/j.gca.2012.03.005.
- Farley KA, Mukhopadhyay S, Isozaki Y, Becker L, Poreda RJ (2001). "An extraterrestrial impact at the Permian–Triassic boundary?". Science. 293 (5539): 2343a–2343. doi:10.1126/science.293.5539.2343a. PMID 11577203.
- Koeberl K, Farley KA, Peucker-Ehrenbrink B, Sephton MA (2004). "Geochemistry of the end-Permian extinction event in Austria and Italy: No evidence for an extraterrestrial component". Geology. 32 (12): 1053–1056. Bibcode:2004Geo....32.1053K. doi:10.1130/G20907.1.
- Romano, Marco; Bernardi, Massimo; Petti, Fabio Massimo; Rubidge, Bruce; Hancox, John; Benton, Michael James (November 2020). "Early Triassic terrestrial tetrapod fauna: a review". Earth-Science Reviews. 210: 103331. Bibcode:2020ESRv..21003331R. doi:10.1016/j.earscirev.2020.103331. S2CID 225066013. Retrieved 12 January 2023.
- Rampino M, Caldeira K, Zhu Y (December 2020). "A 27.5 My underlying periodicity detected in extinction episodes of non-marine tetrapods". Historical Biology. 33 (11): 3084–3090. doi:10.1080/08912963.2020.1849178. S2CID 230580480.
- Gillman M, Erenler H (2008). "The galactic cycle of extinction" (PDF). International Journal of Astrobiology. 7 (1): 17–26. Bibcode:2008IJAsB...7...17G. CiteSeerX 10.1.1.384.9224. doi:10.1017/S1473550408004047. S2CID 31391193.
- Overholt AC, Melott AL, Pohl M (10 November 2009). "Testing the Link Between Terrestrial Climate Change and Galactic Spiral Arm Transit". The Astrophysical Journal. 705 (2): L101–L103. arXiv:0906.2777. Bibcode:2009ApJ...705L.101O. doi:10.1088/0004-637X/705/2/L101. S2CID 734824.
- Powell CS (1 October 2001). "20 Ways the World Could End". Discover Magazine. Retrieved 29 March 2011.
- Podsiadlowski P, Mazzali PA, Nomoto K, Lazzati D, Cappellaro E (2004). "The Rates of Hypernovae and Gamma-Ray Bursts: Implications for Their Progenitors". Astrophysical Journal Letters. 607 (1): L17. arXiv:astro-ph/0403399. Bibcode:2004ApJ...607L..17P. doi:10.1086/421347. S2CID 119407415.
- Melott, Adrian L.; Lieberman, B. S.; Laird, Claude M.; Martin, L. D.; Medvedev, M. V.; Thomas, Brian C.; Cannizzo, John K.; Gehrels, Neil; Jackman, Charles H. (5 August 2004). "Did a gamma-ray burst initiate the late Ordovician mass extinction?". International Journal of Astrobiology. 3 (2): 55–61. arXiv:astro-ph/0309415. Bibcode:2004IJAsB...3...55M. doi:10.1017/S1473550404001910. hdl:1808/9204. S2CID 13124815. Retrieved 27 December 2022.
- Melott AL, Thomas BC (2009). "Late Ordovician geographic patterns of extinction compared with simulations of astrophysical ionizing radiation damage". Paleobiology. 35 (3): 311–20. arXiv:0809.0899. Bibcode:2009Pbio...35..311M. doi:10.1666/0094-8373-35.3.311. S2CID 11942132.
- Fields, Brian D.; Melott, Adrian L.; Ellis, John; Ertel, Adrienne F.; Fry, Brian J.; Lieberman, Bruce S.; Liu, Zhenghai; Miller, Jesse A.; Thomas, Brian C. (1 September 2020). "Supernova triggers for end-Devonian extinctions". Proceedings of the National Academy of Sciences. 117 (35): 21008–21010. arXiv:2007.01887. Bibcode:2020PNAS..11721008F. doi:10.1073/pnas.2013774117. ISSN 0027-8424. PMC 7474607. PMID 32817482.
- Mayhew PJ, Jenkins GB, Benton TG (January 2008). "A long-term association between global temperature and biodiversity, origination and extinction in the fossil record". Proceedings. Biological Sciences. 275 (1630): 47–53. doi:10.1098/rspb.2007.1302. PMC 2562410. PMID 17956842.
- Knoll AH, Bambach RK, Canfield DE, Grotzinger JP (July 1996). "Comparative Earth History and Late Permian Mass Extinction". Science. 273 (5274): 452–457. Bibcode:1996Sci...273..452K. doi:10.1126/science.273.5274.452. PMID 8662528. S2CID 35958753.
- Ward PD, Botha J, Buick R, De Kock MO, Erwin DH, Garrison GH, et al. (February 2005). "Abrupt and gradual extinction among Late Permian land vertebrates in the Karoo basin, South Africa". Science. 307 (5710): 709–714. Bibcode:2005Sci...307..709W. CiteSeerX 10.1.1.503.2065. doi:10.1126/science.1107068. PMID 15661973. S2CID 46198018.
- Kiehl JT, Shields CA (September 2005). "Climate simulation of the latest Permian: Implications for mass extinction". Geology. 33 (9): 757–760. Bibcode:2005Geo....33..757K. doi:10.1130/G21654.1.
- Hecht J (26 March 2002). "Methane prime suspect for greatest mass extinction". New Scientist.
- Jenkyns HC (1 March 2010). "Geochemistry of oceanic anoxic events". Geochemistry, Geophysics, Geosystems. 11 (3): Q03004. Bibcode:2010GGG....11.3004J. doi:10.1029/2009GC002788. ISSN 1525-2027. S2CID 128598428.
- Qiu, Zhen; Zou, Caineng; Mills, Benjamin J. W.; Xiong, Yijun; Tao, Huifei; Lu, Bin; Liu, Hanlin; Xiao, Wenjiao; Poulton, Simon W. (5 April 2022). "A nutrient control on expanded anoxia and global cooling during the Late Ordovician mass extinction". Communications Earth & Environment. 3 (1): 82. Bibcode:2022ComEE...3...82Q. doi:10.1038/s43247-022-00412-x. S2CID 247943064. Retrieved 12 January 2023.
- Zou, Caineng; Qiu, Zhen; Poulton, Simon W.; Dong, Dazhong; Wang, Hongyan; Chen, Daizhou; Lu, Bin; Shi, Zhensheng; Tao, Huifei (2018). "Ocean euxinia and climate change "double whammy" drove the Late Ordovician mass extinction" (PDF). Geology. 46 (6): 535–538. Bibcode:2018Geo....46..535Z. doi:10.1130/G40121.1. S2CID 135039656.
- Men, Xin; Mou, Chuanlong; Ge, Xiangying (1 August 2022). "Changes in palaeoclimate and palaeoenvironment in the Upper Yangtze area (South China) during the Ordovician–Silurian transition". Scientific Reports. 12 (1): 13186. Bibcode:2022NatSR..1213186M. doi:10.1038/s41598-022-17105-2. PMC 9343391. PMID 35915216.
- Bond, David P. G.; Zatoń, Michał; Wignall, Paul B.; Marynowski, Leszek (11 March 2013). "Evidence for shallow-water 'Upper Kellwasser' anoxia in the Frasnian–Famennian reefs of Alberta, Canada". Lethaia. 46 (3): 355–368. doi:10.1111/let.12014. Retrieved 12 January 2023.
- Algeo, T.J.; Scheckler, S. E. (1998). "Terrestrial-marine teleconnections in the Devonian: links between the evolution of land plants, weathering processes, and marine anoxic events". Philosophical Transactions of the Royal Society B: Biological Sciences. 353 (1365): 113–130. doi:10.1098/rstb.1998.0195. PMC 1692181.
- David P. G. Bond; Paul B. Wignalla (2008). "The role of sea-level change and marine anoxia in the Frasnian-Famennian (Late Devonian) mass extinction" (PDF). Palaeogeography, Palaeoclimatology, Palaeoecology. 263 (3–4): 107–118. Bibcode:2008PPP...263..107B. doi:10.1016/j.palaeo.2008.02.015.
- Zhang, Bolin; Wignall, Paul B.; Yao, Suping; Hu, Wenxuan; Liu, Biao (January 2021). "Collapsed upwelling and intensified euxinia in response to climate warming during the Capitanian (Middle Permian) mass extinction". Gondwana Research. 89: 31–46. Bibcode:2021GondR..89...31Z. doi:10.1016/j.gr.2020.09.003. S2CID 224981591. Retrieved 30 September 2022.
- Zhang, Bolin; Yao, Suping; Hu, Wenxuan; Ding, Hai; Liu, Bao; Ren, Yongle (1 October 2019). "Development of a high-productivity and anoxic-euxinic condition during the late Guadalupian in the Lower Yangtze region: Implications for the mid-Capitanian extinction event". Palaeogeography, Palaeoclimatology, Palaeoecology. 531: 108630. Bibcode:2019PPP...53108630Z. doi:10.1016/j.palaeo.2018.01.021. S2CID 133916878. Retrieved 17 November 2022.
- Bond, David P. G.; Wignall, Paul B.; Grasby, Stephen E. (30 August 2019). "The Capitanian (Guadalupian, Middle Permian) mass extinction in NW Pangea (Borup Fiord, Arctic Canada): A global crisis driven by volcanism and anoxia". Geological Society of America Bulletin. 132 (5–6): 931–942. doi:10.1130/B35281.1. S2CID 199104686. Retrieved 29 November 2022.
- Kump, Lee; Alexander Pavlov; Michael A. Arthur (2005). "Massive release of hydrogen sulfide to the surface ocean and atmosphere during intervals of oceanic anoxia". Geology. 33 (5): 397–400. Bibcode:2005Geo....33..397K. doi:10.1130/G21295.1.
- Hülse, Dominik; Lau, Kimberly V.; Van de Velde, Sebastiaan J.; Arndt, Sandra; Meyer, Katja M.; Ridgwell, Andy (28 October 2021). "End-Permian marine extinction due to temperature-driven nutrient recycling and euxinia". Nature Geoscience. 14 (11): 862–867. Bibcode:2021NatGe..14..862H. doi:10.1038/s41561-021-00829-7. S2CID 240076553. Retrieved 12 January 2023.
- Schobben, Martin; Foster, William J.; Sleveland, Arve R. N.; Zuchuat, Valentin; Svensen, Henrik H.; Planke, Sverre; Bond, David P. G.; Marcelis, Fons; Newton, Robert J.; Wignall, Paul B.; Poulton, Simon W. (17 August 2020). "A nutrient control on marine anoxia during the end-Permian mass extinction". Nature Geoscience. 13 (9): 640–646. Bibcode:2020NatGe..13..640S. doi:10.1038/s41561-020-0622-1. S2CID 221146234. Retrieved 12 January 2023.
- Atkinson, J. W.; Wignall, Paul B. (15 August 2019). "How quick was marine recovery after the end-Triassic mass extinction and what role did anoxia play?". Palaeogeography, Palaeoclimatology, Palaeoecology. 528: 99–119. Bibcode:2019PPP...528...99A. doi:10.1016/j.palaeo.2019.05.011. S2CID 164911938. Retrieved 20 December 2022.
- Long JA, Large RR, Lee MS, Benton MJ, Danyushevsky LV, Chiappe LM, et al. (2015). "Severe selenium depletion in the Phanerozoic oceans as a factor in three global mass extinction events". Gondwana Research. 36: 209–218. Bibcode:2016GondR..36..209L. doi:10.1016/j.gr.2015.10.001. hdl:1983/68e97709-15fb-496b-b28d-f8ea9ea9b4fc.
- Watson AJ (December 2016). "Oceans on the edge of anoxia". Science. 354 (6319): 1529–1530. Bibcode:2016Sci...354.1529W. doi:10.1126/science.aaj2321. hdl:10871/25100. PMID 28008026. S2CID 206653923.
- Berner RA, Ward PD (1 January 2006). "Positive Reinforcement, H2S, and the Permo-Triassic Extinction: Comment and Reply: COMMENT". Geology. 34 (1): e100. Bibcode:2006Geo....34E.100B. doi:10.1130/G22641.1.
- Kump LR, Pavlov A, Arthur MA (2005). "Massive release of hydrogen sulfide to the surface ocean and atmosphere during intervals of oceanic anoxia". Geology. 33 (5): 397–400. Bibcode:2005Geo....33..397K. doi:10.1130/g21295.1. Summarised by Ward (2006).
- Wilde P, Berry WB (1984). "Destabilization of the oceanic density structure and its significance to marine "extinction" events". Palaeogeography, Palaeoclimatology, Palaeoecology. 48 (2–4): 143–62. Bibcode:1984PPP....48..143W. doi:10.1016/0031-0182(84)90041-5.
- Wei Y, Pu Z, Zong Q, Wan W, Ren Z, Fraenz M, et al. (1 May 2014). "Oxygen escape from the Earth during geomagnetic reversals: Implications to mass extinction". Earth and Planetary Science Letters. 394: 94–98. Bibcode:2014E&PSL.394...94W. doi:10.1016/j.epsl.2014.03.018 – via NASA ADS.
- "Speculated Causes of the Permian Extinction". Hooper Virtual Paleontological Museum. Retrieved 16 July 2012.
- Smith, Felisa A.; et al. (20 April 2018). "Body size downgrading of mammals over the late Quaternary". Science. 360 (6386): 310–313. Bibcode:2018Sci...360..310S. doi:10.1126/science.aao5987. PMID 29674591.
- Ceballos G, Ehrlich PR, Dirzo R (July 2017). "Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines". Proceedings of the National Academy of Sciences of the United States of America. 114 (30): E6089–E6096. Bibcode:2017PNAS..114E6089C. doi:10.1073/pnas.1704949114. PMC 5544311. PMID 28696295.
- Franck S, Bounama C, von Bloh W (2006). "Causes and Timing of Future Biosphere Extinction" (PDF). Biogeosciences. 3 (1): 85–92. Bibcode:2006BGeo....3...85F. doi:10.5194/bg-3-85-2006. S2CID 129600368.
- Ward P, Brownlee D (December 2003). The Life and Death of Planet Earth: How the New Science of Astrobiology Charts the Ultimate Fate of Our World. Henry Holt and Co. pp. 132, 139, 141. ISBN 978-0-8050-7512-0 – via Google Books.
- Quammen D (October 1998). "Planet of Weeds" (PDF). Harper's Magazine. Retrieved 15 November 2012.
- "Evolution imposes 'speed limit' on recovery after mass extinctions". ScienceDaily. 8 April 2019. Retrieved 7 September 2019.
- Lehrmann DJ, Ramezani J, Bowring SA, Martin MW, Montgomery P, Enos P, et al. (December 2006). "Timing of recovery from the end-Permian extinction: Geochronologic and biostratigraphic constraints from south China". Geology. 34 (12): 1053–1056. Bibcode:2006Geo....34.1053L. doi:10.1130/G22827A.1.
- Sahney S, Benton MJ (April 2008). "Recovery from the most profound mass extinction of all time". Proceedings. Biological Sciences. 275 (1636): 759–765. doi:10.1098/rspb.2007.1370. PMC 2596898. PMID 18198148.
- Sidor CA, Vilhena DA, Angielczyk KD, Huttenlocker AK, Nesbitt SJ, Peecook BR, et al. (May 2013). "Provincialization of terrestrial faunas following the end-Permian mass extinction". Proceedings of the National Academy of Sciences of the United States of America. 110 (20): 8129–8133. Bibcode:2013PNAS..110.8129S. doi:10.1073/pnas.1302323110. PMC 3657826. PMID 23630295.
- Cascales-Miñana B, Cleal CJ (2011). "Plant fossil record and survival analyses". Lethaia. 45: 71–82. doi:10.1111/j.1502-3931.2011.00262.x.
Further reading
- Brannen P (2017). The Ends of the World: Volcanic Apocalypses, Lethal Oceans, and Our Quest to Understand Earth's Past Mass Extinctions. Harper Collins. ISBN 978-0-06-236480-7.
External links
- "Calculate the effects of an impact". Lunar and Planetary Laboratory. Tucson, AZ: University of Arizona.
- "Species Alliance". – nonprofit organization producing a documentary about mass extinction titled "Call of Life: Facing the Mass Extinction"
- "Interstellar dust cloud-induced extinction theory". space.com. 4 March 2005.
- "Sepkoski's Global Genus Database of Marine Animals". Geology. University of Wisconsin. – Calculate extinction rates for yourself!


