Mormyroidea
The Mormyroidea (synonymy: Mormyriformes) are a superfamily (formerly an order) of fresh water fishes endemic to Africa that, together with the families Hiodontidae, Osteoglossidae, Pantodontidae and Notopteridae, represents one of the main groups of living Osteoglossiformes.[1] They stand out for their use of weak electric fields, which they use to orient themselves, reproduce, feed, and communicate.[2][3]
| Mormyroidea | |
|---|---|
 | |
| Elephantnose fish | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Order: | Osteoglossiformes |
| Superfamily: | Mormyroidea |
There is no consensus regarding its superior biological classification as some experts point out that it belongs to the suborder Osteoglossoidei, while others to the Notopteroidei. In either case, the mormyriformes include the gymnarchids and mormyrids[4] and represent the largest superfamily within the order Osteoglossiformes with about two hundred and thirty-three subordinate taxa[5] that are distributed across various watersheds existing throughout tropical Africa south of the Sahara, including the Nile,[6] Turkana, Gambia, and northern South Africa.[7][8][9]
These fish have a large brain and an unusual intelligence,[10] they feed on benthic and allochthonous invertebrates, as well as some crustaceans found in marshy and sandy areas of rivers and lakes.[11] Most of its species are sociable, and although their reproductive form is little known, they generally reproduce during the rainy season and their electrical organs transmit signals with the capacity to influence their reproductive and hormonal behavior.[12][13]
According to the International Union for Conservation of Nature (IUCN), the conservation status of 66.7% of the species is Least Concern and 10.8% is Threatened Species.[14] Furthermore, according to the same institution, the extinction rate of the taxon — at least in the northern region of the African continent — reaches 44.4%, while 55.6% of the individuals are threatened.[15]
Etymology
The term Mormyriformes derives from Greek mormyros, μορμύρος, μόρμυρος, a species of fish that would probably be Lithognathus mormyrus, and from Latin forma, with the same connotation as the English form.[16][17]
Its synonymy Mormyridae is considered the valid taxonomic status according to the Integrated Taxonomic Information System or ITIS of North America.[18] Although several authors included it within the taxonomic category "order" until after the second half of the 20th century,[19] it is considered as a "superfamily" since the mid 1990s.[20]
Distribution and ecology
Ecology
This superfamily has a wide adaptability and can be found in freshwater river systems "with high concentrations of suspended solids and reduced transparency",[21] a water hardness of up to 20 °dH[22] and a salinity level of less than 1 %.[23] The habitats of these fishes are dominated by mud and/or sandy substrates, plant debris, marginal grasses, filamentous algae or clumps of aquatic plants,[23] while the watercourse can be variable: there are species that inhabit barely torrential waters and others in basins where the water flow is high although with presence of pools and rocks that provide areas with lower streamflow.[23]
Distribution
These fishes are distributed in various rivers, lakes, and swamps in Africa (south of the Sahara), the Nile, and the higher temperature sectors of South Africa,[9] with an area of approximately 14 080 000 km².[24]
1n 1909, George Albert Boulenger visited the Congo and described forty-seven endemic species, fourteen of them in northern West Africa in the Congo region, eight in West Africa on the Congo and other rivers, seven in the Nile, six in both the Nile and Lake Chad and the Niger and Senegal, and two in Lake Victoria.[25]
In 2003, Didier Paugy, Christian Lévêque, and Guy G. Teugels published a regional synthesis of West African fishes and identified a total of fifteen genera with 41 species in Lower Guinea and fourteen genera with 44 species in West Africa.[26] In 2006, Christian Lévêque and Didier Paugy analyzed the composition of the fish fauna in the most representative rivers and lakes of the main ichthyological provinces of Africa and determined the presence of fifteen species in the Nile, fourteen in Chad, twenty-seven in Niger, sixteen in the Volta River, ten in the Konkouré River, thirteen in the Jong, eight in the Sassandra, ten in the Bandama, fifteen on the Sanaga, twenty-two on the Ogôoué, six on the Ruaha, 109 on the Congo, and ten on the Zambèze.[27] Additionally, within the freshwater or epicontinental species of the richest African aquatic systems, 19 of them are found in the Niger River, 75 in the Congo River and 16 others in the Zambezi River.[21][note 1]
In 2008, Melanie L. J. Stiassny, Guy G. Teugels and Carl Hopkins evaluated the geographic distribution of genera in the subfamily Mormyrinae and indicated that at least fourteen are found in Lower Guinea; the remainder can be found in Congo as in the case of Genomyrus, Angola as in Heteromormyrus, Nilo-Sudan as in Hyperopisus and Cyphomyrus, and South Africa as in Cyphomyrus.[26] In addition, the same authors indicated that at least six species in the subfamily Petrocephalinae are in Lower Guinea,[26] while in 2012, several researchers from the universities of Regensburg and Heidelberg, in conjunction with the South African Institute for Aquatic Biodiversity, indicated the presence of several new species in the rivers Luongo, Lufubu, Zambezi, Boro, Cunene, Thoage, and the Okavango Delta.[23]
Morphology
Sizes and shapes
The superfamily Mormyridae has a high diversity within its more than 200 species and subspecies, with a range of sizes and shapes that varies according to the family of membership and their respective genus. The smallest can measure around 4 cm in their adult stage, while the largest can reach 150 cm,[28] although a specimen belonging to Gymnarchus niloticus which reached a size of 167 cm is known to exist in the Loumbila reserve, near Ouagadougou.[26] Its body has cycloid scales, small eyes — which in the case of mormyrids are covered with skin — and a mouth that is not protractile which may vary depending on the genus:.[26][29]
- The genera Campylomormyrus, Gnathonemus and Mormyrus possess a particularly prominent extending mouth that usually consists of a flexible fleshy elongation attached to the lower jaw and is equipped with touch and probably taste sensors, which is why they are popularly called "elephant-nose fishes".[30]
- The genera Mormyrops, Brienomyrus, Hippopotamyrus, Marcusenius, Petrocephalus, and Pollimyrus possess small barbs and usually lack the extended mouthparts of elephantfishes, hence they are called "Nile river pikes".[28]
- The genus Gymnarchus has a prominent snout with "strong, pointed or notched teeth that line up in a single row on both jaws."[26]
Brain and cerebellum
The brain of this superfamily is one of the largest among fishes and has a body-proportional size comparable to that of humans,[31] with a brain-to-body mass ratio ranging from 1/52 to 1/82, and possibly associated with the ability to interpret bioelectrical signals.[32] Since the pioneering work of Michael Pius Erdl in 1846,[33] several researchers have made efforts toward describing the development of this organ and its functionality.
Thus, based on the analysis of larvae and embryos of Pollimyrus (Marcusenius) Isidori, it is known that "the brain develops very rapidly: the corpus cerebelli (c.cer) and cerebellar structures, i.e. eminentia granularis (e. gr), lobus caudalis (lc) and transitorius (lt), lobi lineae lateralis (lll), are formed in 40 days, whereas valve development needs 180."[34] They possess a hypertrophy in the cerebellum, which the literature refers to as mormyrocerebellum or gigantocerebellum, "probably related to his unique electrogenic and electroreceptive abilities"[35] and to the large size of the valve,[36] which in turn relates to the electrosensory system present in these fish.[37]
It has been found that for species living in oxygen-deficient aquatic environments, they protect their brains from damage caused by hypoxia through efficient utilization of existing oxygen.[35] Furthermore, among its species, Gnathonemus petersii was found to hold the record among vertebrates — including humans — as the one whose brain consumes at least 60 % of all body oxygen.[38]
Electric organs

Mormyriformes can produce weak electric signals with a specialized organ discovered in 1951 by the British-Ukrainian researcher Hans Lissmann after observing a live specimen of Gymnarchus niloticus.[40] Such an organ is evolutionarily derived from muscle cells, and there is a degree of convergent evolution in form and function with the Gymnotiformes of South America, especially in the sensory apparatus for detecting and processing electrical signals involving electrocommunication and electrolocation processes.[41][26]
Ampullary organ
Ampullae of Lorenzini are electroreceptors "extremely sensitive to low-frequency fields of biotic or abiotic origin and are generally used in the context of passive electrolocation",[39] with a high sensitivity of 0.01 mV/cm and sensitive to DC fields or frequencies lower than 50 Hz.[42]
Tuberous organs
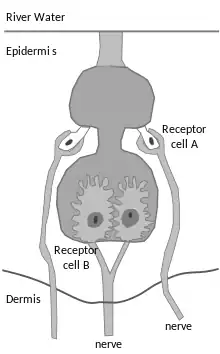
These fish have two types of tuberous electroreceptor:[note 2] the Knollenorgan[43] and the Mormyromast.[44][45] Both organs are found in adult individuals, where they are lightly covered by epithelial cells and skin, while their sensitivity ranges from 0.1 mV and 10 mV/cm/ Tens of Hz up to more than a kHz.[42]
The knollenorgan was first described in 1921 as an epidermal organ by the German anatomist Victor Franz for a Marcusenius species, although without discovering its function.[46] It is composed of a set of receptor cells that can reach between 40 and 60 microns in diameter; these are located under the skin and have a sensitivity of approximately 0.1 mV/cm.[47]
The mormyromast appeared under the name Schnauzenorgan ('Snout organ') in a paper by Walter Stendell in 1914, where he described it as a combination of the sensory and glandular apparatus for a species of Mormyrus.[48][46] The larval form of the receptor, the promormyromast, differs from the adult's in cellular composition, distribution in the epidermis and innervation.[48][49] This organ is one of the most abundant in mormyriforms, with a high concentration of electroreceptors in the epidermis per cm²: for example, for Gnathonemus petersii there are about 2000 per cm², versus a maximum of 50 receptors per cm² for ampullary organs and knollenorgans.[48] In 2009, Engelmann and colleagues showed that G. petersii actively moves its elongated "elephantnose" chin to localise prey accurately, i.e. this species has an active sensory-motor loop that links electroreception to "active motor exploration of the environment".[39][50]
Behavior
Communication
Some species of mormyriformes, predominantly in the mormyridae family, are sociable, attentive and intelligent, whereas the gymnarchidae are solitary, unintelligent and even aggressive.[51][52][22]
These fish have the ability to produce and analyze weak electric fields through a specialized organ,[40] (electric organ discharges), which Lissmann and coworkers first analyzed in 1958 through several experiments.[53][54] Such electric fields provide these fish with a specialized sensory system for communication and orientation.[55]
EODs are useful for orientation, finding food and communication,[2][3][56][57] whose frequency is variable;[58] fields allow them to locate objects and react to other animals in turbid waters — or waters of reduced transparency — where their vision is affected by the presence of organic matter and suspended solids.[59]
This system is a recurrent subject of scientific research, particularly in the field of inter- (and intra-) species communication, as well as in studies of electrophysiology and behavior.[60]
EODs are often pulsatile, with frequencies exceeding 130 Hz for the most aggressive,[58] except in the case of Gymnarchus, whose electrical organs discharge at between 300 and 500 Hz under normal conditions, giving a sinusoidal appearance to the discharge.[61]
Feeding

Feeding generally consists of small invertebrates buried in muddy substrates, marshy areas and sandy riverbank areas; thus, throughout the year certain species consume some crustaceans found on the banks of running rivers,[11] larvae and pupas of chironomids, coarse particulate organic matter and mud/sand.[62][63][64]
The diet of this superfamily may change depending on seasonal variations in rainfall,[65] since while in dry season some species supplement their diet with high amounts of larvae of Trichoptera, in rainy season this includes Ephemeroptera.[62][64]
The trophic flexibility of mormyriformes allows them to survive in the diverse ecosystems they inhabit,[66][64] so it is possible to find in their diet from benthic invertebrates to allochthonous, while in some species there are no differences regarding their diet if sex and/or season are considered.[67]
Reproduction
The breadth of species within the superfamily Mormyridae results in a paucity of information on their reproduction and intergeneric relationships, which is limited in both behavioral and biological terms.[68] However, and in comparison to the electric fishes Gymnotiformes of South America,[69] there is a greater understanding regarding their reproduction: it is known that much of the species reproduces in the rainy season[13] and their specialized electrical organs generate pulses that form an electromotor system that transmits electrical signals with the ability to influence their reproductive and hormonal behavior,[70][13] such as in 11-ketotestosterone levels.[13][note 3]
Courtship takes place at the beginning of the rainy season, as the water level within the riverine sectors increases and decreases its conductivity —and the pH-value—; some species migrate towards the flooded areas,[71] and during the mating season produce certain sounds and electrical discharge patterns.[72] Other specimens build nests (as in the case of Pollimyrus and Gymnarchus which make floating nests of plant material)[22] or migrate seeking shallow, non-torrential areas with sandy loamy bottoms (such as Brienomyrus longianalis),[71] while some others dig holes in the ground to spawn.[12]
There is little information regarding the larval and egg morphology of this superfamily, a situation that is repeated in virtually the entire osteoglossomorpha superorder.[73] In this regard, there are data for some species such as Hyperopisus bebe, Pollimyrus adspersus, Mormyrus rume proboscirostris, Campylomormyrus tamandua, Hippopotamyrus pictus and Petrocephalus soudanensis, although only in the case of Pollimyrus isidor an analysis on its embryonic and larval development is appreciated.[74]
Eggs are variable in size, with a probable maximum size of about 10 millimeters in diameter as occurs in gymnarchids.[75] After laying the eggs, both the male and female guard the nest, while after eighteen days, they hatch and the larvae swim freely.[76]
Classification
The first respectable classification for some of the species in this superfamily appeared in Volume I of the tenth edition of Linnaeus's Systema naturae in 1758[note 4] which, based on John Ray's Synopsis methodica Animalium (1693),[77] included the genus Mormyrus within the order Branchiostegui.[78] Since this work, various changes occurred within the international zoological literature, and it was not until the appearance of the International Code of Zoological Nomenclature in 1905 where the subordination of taxa followed a homogeneous pattern.[79]
Thus, while in the 1950s the mormyriformes included the families Mormyridae, Notopteridae and Hiodontidae within the Clupeiformes group,[80] as early as the 1960s was regarded as an order containing the families Mormyridae and Gymnarchidae.[81] In 1972, Louis Taverne proposed the inclusion as a suborder to the Mormyridae and Gymnarchoidea, which in turn agglutinated the families Mormyridae — with the subfamilies Petrocephalinae and Mormyrinae — and Gymnarchidae and Gymnarchidae respectively.[82] His analysis considered osteological and external morphological characteristics, in addition to proposing a phylogenetic tree and reaffirming its inclusion within the osteoglossomorphs,[82] a superorder of primarily marine teleostei fishes proposed by Peter Humphry Greenwood, Donn E. Rosen, Stanley H. Weitzman and George S. Myers in Phyletic Studies of Teleostean Fishes, with a Provisional Classification of Living Forms of 1966.[81]
According to the Integrated Taxonomic Information System, the superfamily Mormyridae includes 233 subordinate taxa, with two families, twenty genera, 186 species, and 15 subspecies;[5] such a classification maintains the logic proposed by Taverne.[83][84][85][82]
Family Gymnarchidae (Bleeker, 1859)
The Gymnarchidae are a family containing the genus Gymnarchus — a name proposed by Georges Cuvier in the second edition of his Le Règne Animal in 1829[17] — and contains a single species: Gymnarchus niloticus, which is found exclusively in swamps and vegetated edges in the Nile, Turkana, Chad, Niger, Volta, Senegal, and Gambia.
The scientific name of this family comes from Pieter Bleeker, who first used it in a series of articles in the journal Natuurkundig tijdschrift voor Nederlandsch Indië of 1859.
Family Mormyridae (Bonaparte, 1832)

The Mormyridae represent the most extensive family in that order with about two hundred species distributed throughout various river basins of tropical Africa south of the Sahara and the Nile.[6]
The scientific name of this family comes from Charles Lucien Bonaparte, who first proposed it in Iconografia della Fauna Italica per le quattro Classi degli Animali Vertebrati of 1832, while the subfamilies received their designation from Taverne in Ostéologie des genres Mormyrus Linné, Mormyrops Müller, Hyperopisus Gill, Isichthys Gill, Myomyrus Boulenger, Stomatorhinus Boulenger et Gymnarchus Cuvier. Considérations générales sur la systématique des poissons d l'ordre des mormyriformes of 1972:[82]
- The subfamily Petrocephalinae contains only one genus of the family Mormyridae (Petrocephalus), so it forms a monophyletic group within it, with approximately forty species and subspecies, some of them discovered only in 2012.[23][26]
- The subfamily Mormyrinae contains almost all the genera of the family Mormyridae, making it one of the largest subfamilies in the order Osteoglossiformes. There are approximately one hundred and seventy species in nineteen genera:[26]
- Boulengeromyrus (Taverne y Géry, 1968)
- Brevimyrus (Taverne, 1971)
- Brienomyrus (Taverne, 1971)
- Campylomormyrus (Bleeker, 1874)
- Cyphomyrus (Pappenheim, 1906)
- Genyomyrus (Boulenger, 1898)
- Gnathonemus (Gill, 1863)
- Heteromormyrus (Steindachner, 1866)
- Hippopotamyrus (Pappenheim, 1906)
- Hyperopisus (Gill, 1862)
- Isichthys (Gill, 1863)
- Ivindomyrus (Taverne y Géry, 1975)
- Marcusenius (Gill, 1862)
- Mormyrops (J. P. Müller, 1843)
- Mormyrus (Linnaeus, 1758)
- Myomyrus (Boulenger, 1898)
- Oxymormyrus (Bleeker, 1874)
- Paramormyrops (Taverne, Thys van den audenaerde y Heymer, 1977)
- Pollimyrus (Taverne, 1971)
- Stomatorhinus (Boulenger, 1898)
Particularly for the genus Campylomormyrus, at least fourteen species are accepted — by virtue of the taxonomic revision carried out by Poll — although their number is still subject to debate since the exact number accepted in the literature varies from three to sixteen, by virtue of the morphological analyses carried out by their authors.[86]
Phylogeny
There is no consensus regarding the origin and diversification of the species belonging to this superfamily, since while some suggest that it appeared before the separation between Africa and South America, others indicate that it was later.[87]
One of the oldest fossils corresponds to a partial dentition of a species of Gymnarchus that lived at the end of the Eocene — about 37 million years ago — in the Faiyum oasis of Egypt,[88] and succeeding remains of the genus Hyperopisus in Pliocene deposits in Wadi El Natrun of Egypt and from the Plio-Pleistocene — more recent than 5 million years ago — in Uganda, Lake Edward, and the Semliki River in Congo.[89][90]
In 1999, it was estimated on the basis of Mitochondrial DNA from thirteen species — two Petrocephalinae, one Gymnarchidae and ten Mormyrinae — that the core group of the mormyriformes could be between 60.69 and 71.98 million years old,[68] while a year later, a date of 242 ± 23 million years was determined, though through the construction of a molecular phylogeny with nucleotide sequences of two mitochondrial genes from 12 species of Osteoglossiformes.[91] In 2009, a new estimate was made with the species Brienomyrus Niger and Gnathonemus petersii, which determined 162 ± 24 million years.[92]
The following cladogram shows the relationship between the different families of Mormyriformes outlined by Sébastien Lavoué and colleagues:[93][94]
| Mormyroidea |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Threats and protection
According to the information available for the 178 species assessed by the IUCN, the conservation status of the species associated with this superfamily is heterogeneous: 118 can be categorized as "least concern (LC or LR/lc)", 15 as "vulnerable (VU)", 3 as "near threatened (NT or LR/nt)" and 4 as "endangered (EN)".[14]
The global status of mormyrids and gymnarchids is unclear, since there are accurate and systematic data only for the North African region, where the extinction rate of the former taxon in 2011 is estimated at 44.4% with 55.6% of individuals in danger,[15] while for the latter, the IUCN recommends greater efforts to census its population and the threats that afflict it.[95]
The main threats depend on the geographic area, country or river basin:
- Several species have economic importance in the areas where they inhabit, including Hyperopisus bebe,[66] Mormyrus rume, Gymnarchus niloticus[96] or the Campylomormyrus bredoi,[97] whose overfishing with trawl nets elevated it to vulnerable status. According to estimates by the Fisheries and Aquaculture Department of the FAO, catches as of 2009 worldwide reached 35 685 tons of mormyrids and 13 901 tons of gymnarchids, representing an increase of 183.9% and 52.8% respectively since 2002.[98]
- Externalities due to mining activities on the banks of various rivers are also a cause of threat, observable in the case of Ivindomyrus opdenboschi in the Ntem and Ivindo rivers[99] or the Marcusenius cuangoanus in the banks of the Kasai River.[100]
- Habitat loss and degradation due to agriculture, urban development, and deforestation is also a source of threat, as in the case of Marcusenius brucii,[101] the Marcusenius abadii in the Black Volta or the Oti/Pendjari River,[102] the Marcusenius furcidens in the Tano River[103] or the Mormyrus cyaneus in the Bas-Congo.[104]
On the other hand, the conservation actions implemented are scarce or non-existent for most species, of low impact, and with unknown results.[14]
Notes
- From the point of view of their fish richness, the rivers Niger, Nile, Congo, Zambezi and the great lakes of Central Africa are the most relevant.
- Also present in Gymnotiformes fishes.
- 11-ketotestosterone functions as the endogenous sex hormone androgenic in fish.
- The tenth edition of Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis is considered the starting point of zoological nomenclature.
References
- Wilson, M. V. H.; Murray, A. M. (2008). "Osteoglossomorpha: phylogeny, biogeography, and fossil record and the significance of key African and Chinese fossil taxa". Geological Society, London, Special Publications. 295: 185–219. doi:10.1144/SP295.12. S2CID 84322935.
- Hollmann, Michael; Engelmann, Jacob; von der Emde, G. (2008). "Distribution, density and morphology of electroreceptor organs in mormyrid weakly electric fish: anatomical investigations of a receptor mosaic". Journal of Zoology. 276 (2): 149–158. doi:10.1111/j.1469-7998.2008.00465.x. ISSN 1469-7998.
- Pusch, R.; von der Emde, G.; Hollmann, M.; Bacelo, J.; Nöbel, S.; Grant, K.; Engelmann, J. (2008). "Active sensing in a mormyrid fish – Electric images and peripheral modifications of the signal carrier give evidence of dual foveation". Journal of Experimental Biology. 211 (Pt 6): 921–934. doi:10.1242/jeb.014175. PMID 18310118. S2CID 1148095.
- Hopkins, Carl D. (1999). "Signal Evolution in Electric Communication". The Design of Animal Communication. MIT Press. ISBN 978-02-6258-223-0.
- "Mormyroidea: taxa subordinado". Canadian Biodiversity Information Facility (CBIF). 14 December 2021.
- Skelton, Paul Harvey (2001). A complete guide to the freshwater fishes of Southern Africa. South Africa: Struik Publishers. pp. 395. ISBN 978-18-6872-643-1.
- Witte, Frans; van Oijen, Martien J.P.; Sibbing, Ferdinand A. (2009). "Fish Fauna of the Nile". The Nile: Origin, Environments, Limnology and Human Use. Belgium: Springer. pp. 818. ISBN 978-14-0209-725-6.
- Seegers, Lothar (1996). The fishes of the Lake Rukwa drainage. Belgium: Koninklijk Museum voor Midden-Afrika. p. 407. ISBN 978-90-7589-403-5.
- Berra, Tim M. (2007). Freshwater Fish Distribution. Chicago: University of Chicago Press. pp. 615. ISBN 978-02-2604-442-2.
- Tiwari, S. K. (1999). Animal Kingdom of the World, Volume 2: Faunal Regions of the world. New Delhi: Sarup & Sons. p. 670. ISBN 978-81-7625-071-9.
- Mikuriya, Beverly A. (1972). "The gross anatomy and microscopic anatomy of the tongue and lower jaw of Gnathonemus petersii (Gthr. 1862) (Mormyridae, Teleostei)". Zoomorphology. 73 (3): 195–208. doi:10.1007/BF00297205. ISSN 0720-213X. S2CID 12988848.
- Sterba, Günther (1999). Süsswasserfische der Welt (in German). Augsburg: Weltbild. p. 914. ISBN 978-38-9350-991-1.
- Simon, Neal (2002). "Hormonal Processes in the Development and Expression of Aggressive Behavior". Hormones, Brain and Behavior. Vol. 1. Academic Press. ISBN 978-00-8053-415-2.
- IUCN (2011). "IUCN Red List of Threatened Species. Version 2011.2". IUCN Red List.
- García, N.; Cuttelod, A.; Abdul Malak, D. (2011). The status and distribution of freshwater biodiversity in northern Africa. Switzerland: IUCN. p. 141. ISBN 978-28-3171-271-0.
- Craig, John (1859). A new universal etymological technological, and pronouncing dictionary of the English language. London: Routledge.
- Cuvier, Georges (1831). "The Animal Kingdom (Vol. II: Reptiles - Fishes)". Foundation for the National History of Animals. London: Henderson.
- "Mormyriformes". Sistema Integrado de Información Taxonómica.
- Joseph S., Nelson (1976). Fishes of the World. New York: John Wiley and Sons. pp. 416. ISBN 0-471-01497-4.
- Joseph S., Nelson (1994). Fishes of the World. New York: John Wiley and Sons. pp. 600. ISBN 0-471-54713-1.
- Granado Lorencio, Carlos (2000). Ecología de los peces: El paradigma de los peces de agua dulce [Ecology of Fishes: The paradigm of freshwater fiches] (in Spanish). Universidad de Sevilla. p. 282. ISBN 978-84-4720-600-1.
- Sandford, Gina (1994). El Libro Completo de Los Peces de Acuario: Guía Completa para Identificar, Escoger y Mantener Especies de Agua Dulce y Marinas [The Complete Book of Aquarium Fishes] (in Spanish). Ediciones AKAL. p. 95. ISBN 978-84-8775-644-3.
- Kramer, Bernd; Bills, Roger; Skelton, Paul; Wink, Michael (2012). "A critical revision of the churchill snoutfish, genus Petrocephalus Marcusen, 1854 (Actinopterygii: Teleostei: Mormyridae), from southern and eastern Africa, with the recognition of Petrocephalus tanensis, and the description of five new species" (PDF). Journal of Natural History. 46 (35–36): 2179–2258. doi:10.1080/00222933.2012.708452. S2CID 85313123.
- del Castillo, Marcos (1986). "Nueva aproximación metodológica al estudio de la biogeografía de los peces epicontinentales". Oecología aquatica (in Spanish). 8: 71–94.
- Banaescu, Petru (1990). Zoogeography of Fresh Waters. Wiesbaden: Seite 63, AULA. ISBN 9783891044810.
- Stiassny, Melanie L.J.; Teugels, Guy G.; Hopkins, Carl (2008). Poissons d'eaux douces et saumâtres de basse Guinée, ouest de l'Afrique centrale, Volume 1 - Volume 42 (PDF) (in English and French). IRD Editions. p. 799. ISBN 978-2-7099-1432-1.
- Lévêque, Christian; Paugy, Didier (2006). "Caractéristiques générales de la faune ichtyologique". Les poissons des eaux continentales africaines. Diversité, écologie, utilisation par l'homme (PDF) (in French). Paris: IRD Éditions. ISBN 2-7099-1589-8.
- "Mormyridae". Mormyridae - African weakly electric fishes. Archived from the original on 18 June 2013.
- Cockerell, T. D. A. "Scales of the Mormyrid Fishes with Remarks on Albula and Elops". Smithsonian Miscellaneous Collections. 56 (3): 1–4.
- Suyehiro, Y. (1942). "A study on the digestive system and feeding habits of fish". Japanese Journal of Zoology. 10 (1): 1–305. ISSN 0044-5118.
- Nelson, Joseph S. (2006). Fishes of the world. Hoboken, New Jersey: John Wiley and Sons. pp. 601. ISBN 978-04-7125-031-9.
- Helfman, Gene S.; Collette, Bruce B.; Facey, Douglas E.; Bowen, Brian W. (2009). The diversity of fishes: Biology, Evolution, and Ecology. Hoboken, New Jersey: John Wiley & Sons. p. 720. ISBN 978-14-0512-494-2.
- Erdl, M.P. (1846). "Über das Gehirn der Fischgattung Mormyrus". Gelehrte Anzeigen der königlichen bayerischen Akademie der Wissenschaften (in German) (22–23): 403–407.
- Haugede-Carre, F.; Kirschbaum, F.; Szabo, T. (1977). "On the development of the gigantocerebellum in the mormyrid fish Pollimyrus (Marcusenius) Isidori". Neuroscience Letters. 6 (2–3): 209–213. doi:10.1016/0304-3940(77)90020-9. ISSN 0304-3940. PMID 19605054. S2CID 40198043.
- Chapman, Lauren J.; Hulen, Kevin G. (2001). "Implications of hypoxia for the brain size and gill morphometry of mormyrid fishes". Journal of Zoology. 254 (4): 461–472. doi:10.1017/S0952836901000966.
- Glickstein, M.; Voogd, J. (2009). "Cerebellum: Evolution and Comparative Anatomy". Encyclopedia of Neuroscience: 743–756. doi:10.1016/B978-008045046-9.00947-5. ISBN 978-0-08-045046-9.
- Wullimann, Mario F.; Rooney, Donal J. (1990). "A direct cerebello-telencephalic projection in an electrosensory mormyrid fish" (PDF). Brain Research. 520 (1–2): 354–357. doi:10.1016/0006-8993(90)91730-5. PMID 1698507. S2CID 12951395.
- Nilsson, Göran E. (1996). "Brain and body oxygen requirements of Gnathonemus petersii, a fish with an exceptionally large brain" (PDF). The Journal of Experimental Biology. 199 (3): 603–607. doi:10.1242/jeb.199.3.603. ISSN 1477-9145. PMID 9318319.
- Engelmann, Jacob; Nöbel, Sabine; Röver, Timo; von der Emde, Gerhard (2009). "The Schnauzenorgan-response of Gnathonemus petersii" (PDF). Frontiers in Zoology. 6 (21): 1–15. doi:10.1186/1742-9994-6-21. PMC 2760544. PMID 19772622.
- Lissmann, H.W. (1951). "Continuous electrical signals from the tail of a fish, Gymnarchus niloticus Cuv". Nature. 167 (4240): 201–202. doi:10.1038/167201a0. PMID 14806425. S2CID 4291029.
- Granado Lorencio, Carlos (2000). Ecología de comunidades: el paradigma de los peces de agua dulce (in Spanish). Seville: Universidad de Sevilla. p. 282. ISBN 978-84-4720-600-1.
- Hopkins, Carl D. "Electroreception". Department of Neurobiology and Behavior, Cornell University.
- Derbin, C.; Szabo, T. (1968). "Ultrastructure of an electroreceptor (Knollenorgan) in the Mormyrid fish Gnathonemus petersii. I.". Journal of Ultrastructure Research. 22 (5): 469–484. doi:10.1016/S0022-5320(68)90035-X. ISSN 0022-5320. PMID 5658648.
- Bell, C. C.; Zakon, H.; Finger, T. E. (1989). "Mormyromast electroreceptor organs and their afferent fibers in mormyrid fish: I. Morphology". The Journal of Comparative Neurology. 286 (3): 391–407. doi:10.1002/cne.902860309. ISSN 0021-9967. PMID 2768566. S2CID 44327227.
- Hara, Toshiaki J.; Zielinski, Barbara (2006). Fish Physiology: Sensory Systems Neuroscience: Sensory Systems Neuroscience. Academic Press. p. 536. ISBN 978-00-8046-961-4.
- Franz, Victor (1921). "Zur mikroskopischen Anatomie der Mormyriden" [On the Microscopic Anatomy of the Mormyrids] (PDF). Zoologisches Jahrbuch (in German). 42: 91–147.
- Hopkins, Carl D. "Knollenorgan Electroreceptors". Department of Neurobiology and Behavior (NB&B), Cornell University.
- Denizot, J. P.; Bensouilah, M.; Roesler, R.; Schugardt, C.; Kirschbaum, F. (2007). "Larval Electroreceptors in the Epidermis of Mormyrid Fish: II. The Promormyromast" (PDF). The Journal of Comparative Neurology. 501 (5): 810–823. doi:10.1002/cne.21278. PMID 17299756. S2CID 15047334. Archived from the original (PDF) on 19 December 2014.
- Denizot, Jean-Pierre; Kirschbaum, Frank; Schugardt, Christian; Bensouilah, Mourad (1998). "Larval electroreceptors indicate a larval electric system in mormyrids". Neuroscience Letters. 241 (2–3): 103–106. doi:10.1016/S0304-3940(98)00030-5. PMID 9507931. S2CID 28490446.
- Engelmann, Jacob; Bacelo, João; Metzen, Michael; Pusch, Roland; Bouton, Beatrice; Migliaro, Adriana; Caputi, Angel; Budelli, Ruben; Grant, Kirsty; von der Emde, Gerhard (20 May 2008). "Electric imaging through active electrolocation: implication for the analysis of complex scenes". Biological Cybernetics. 98 (6): 519–539. doi:10.1007/s00422-008-0213-5. PMID 18491164. S2CID 2975352.
- Anderson, Edward William (1983). Animals as Navigators. Van Nostrand Reinhold. pp. 206. ISBN 978-04-4220-882-0.
- Grzimek, Bernhard (1973). Grzimek's Animal Life Encyclopedia, Volume 4: Fishes. Van Nostrand Reinhold. p. 531.
- Lissmann, Hans W. (1958). "On the function and evolution of electric organs in fish" (PDF). The Journal of Experimental Biology. 35 (1): 156–191. doi:10.1242/jeb.35.1.156.
- Lissmann, Hans W.; Machin, K.E. (1958). "The mechanism of object location in Gymnarchus niloticus and similar fish" (PDF). The Journal of Experimental Biology. 35 (2): 451–86. doi:10.1242/jeb.35.2.451.
- Lavoué, Sébastien; Sullivana, John P.; Hopkins, Carl D. (2008). "Petrocephalus of Odzala offer insights into evolutionary patterns of signal diversification in the Mormyridae, a family of weakly electrogenic fishes from Africa". Journal of Physiology-Paris. 102 (4–6): 322–339. doi:10.1016/j.jphysparis.2008.10.003. ISSN 0928-4257. PMID 18992333. S2CID 2989633.
- Bacelo, Joao; Engelmann, Jacob; Hollmann, Michael; von der Emde, Gerhard; Grant, Kristy (2008). "Functional foveae in an electrosensory system" (PDF). The Journal of Comparative Neurology. 511 (3): 342–359. doi:10.1002/cne.21843. PMID 18803238. S2CID 17186368. Archived from the original (PDF) on 3 March 2016.
- von der Emde, Gerhard; Amey, Monique; Engelmann, Jacon; et al. (2008). "Active Electrolocation in Gnathonemus petersii: Behavior, Sensory Performance and Receptor Systems". Journal of Physiology, Paris. 102 (4–6): 279–290. doi:10.1016/j.jphysparis.2008.10.017. PMID 18992334. S2CID 36652884.
- Bauer, Richard (1974). "Electric Organ Discharge Activity of Resting and Stimulated Gnathonemus petersii (Mormyridae)". Behaviour. 50 (3–4): 306–323. doi:10.1163/156853974x00507. PMID 4447583.
- Greenwood, P. H.; Wilson, M. V. (1998). "Mormyridaes". Encyclopedia of Fishes. San Diego: Academic Press. p. 84. ISBN 978-0-12-374545-3.
- Lavoué, Sébastien; Bigornec, Rémy; et al. (2000). "Phylogenetic Relationships of Mormyrid Electric Fishes (Mormyridae; Teleostei) Inferred from Cytochrome b Sequences". Molecular Phylogenetics and Evolution. 1 (14): 1–10. doi:10.1006/mpev.1999.0687. ISSN 1055-7903. PMID 10631038.
- Bullock, Theodore H.; Behrend, Konstantin; Heiligenberg, Walter (1975). "Comparison of the jamming avoidance responses in Gymnotoid and Gymnarchid electric fish: a case of convergent evolution of behavior and its sensory basis". Journal of Comparative Physiology A: Neuroethology, Sensory, Neural & Behavioral Physiology. 103 (1): 97–121. doi:10.1007/BF01380047. ISSN 0340-7594. S2CID 20094087.
- Blake, B.F. (1977). "Food and feeding of the mormyrid fishes of Lake Kainji, Nigeria, with special reference to seasonal variation and interspecific differences". Journal of Fish Biology. 11 (4): 315–328. doi:10.1111/j.1095-8649.1977.tb04125.x. ISSN 1095-8649.
- Imevbore, A.; Bakere, O. (1970). "Bottom deposits as food of inland water fish. In: Kainji, a Nigerian Man-made Lake". Ecology (Ed. S.A. Visser). 1: 65–85.
- Nwani, Christopher Didigwu; Odoh, Gregory Ejike; et al. (2011). "Food and feeding habits of Gnathonemus petersii (Osteichthyes: Mormyridae) in Anambra River, Nigeria" (PDF). International Aquatic Research. 3 (3): 45–51. Archived from the original (PDF) on 29 November 2014.
- Marrero, Críspulo (1990). "Contribución al estudio de los hábitos alimentarios de los peces tropicales de agua dulce. Alimentación de Rhamphicthys marmoratus Castelnau, 1855 (Teleostei, Gimnotiformes, RHAMPHICTHYDAE) en el bajo llano de Venezuela" (PDF). Memoria, Sociedad de Ciencias Naturales la Salle (in Spanish). 48 (131–134): 131–140.
- Olele, N.F. (2011). "Diet Composition, Length/Weight Relationship and Condition Factor of Hyperopisus bebe occidentalis (Lacepede 1803) Caught in Warri River" (PDF). Journal of Basic and Applied Scientific Research. 1 (9): 998–1005. ISSN 2090-424X. Archived from the original (PDF) on 19 May 2015.
- Nwani, Christopher Didigwu; Eyo, Joseph Effiong; Udeh, Emmanuel Fame (2006). "Food and Feeding Habits of Campylomormyrus tamandua in Anambra River, Nigeria" (PDF). Animal Research International. 3 (1): 410–414. Archived from the original (PDF) on 29 November 2014.
- Alves-Gomes, José A. (1999). "Systematic biology of gymnotiform and mormyriform electric fishes: phylogenetic relationships, molecular clocks and rates of evolution in the mitochondrial rRNA genes" (PDF). Journal of Experimental Biology. 202 (10): 1167–1183. doi:10.1242/jeb.202.10.1167. ISSN 1477-9145. PMID 10210659.
- Moller, Peter (1995). Electric fishes: history and behavior. Chapman & Hall. p. 584. ISBN 978-04-1237-380-0.
- Cabej, Nelson R. (2011). Epigenetic Principles of Evolution. Elsevier. p. 846. ISBN 978-01-2415-851-1.
- Ikomi, R.B. (1996). "Studies on the growth pattern, feeding habits and reproductive characteristics of the mormyrid Brienomyrus longianalis (Boulenger, 1901) in the upper Warri River, Nigeria". Fisheries Research. 26 (1–2): 187–198. doi:10.1016/0165-7836(95)00381-9. ISSN 0165-7836.
- Terleph, Thomas A.; Moller, Peter (2003). "Effects of social interaction on the electric organ discharge in a mormyrid fish, Gnathonemus petersii (Mormyridae, Teleostei)". The Journal of Experimental Biology. 206 (pt 14): 2355–2362. doi:10.1242/jeb.00437. ISSN 0366-0788. PMID 12796452. S2CID 2148613.
- Britz, Ralf (2004). "Egg structure and larval development of Pantodon buchholzi (Teleostei: Osteoglossomorpha), with a review of the data on reproduction and early life history in the other osteoglossomorphs". Ichthyological Exploration of Freshwaters. 15 (3): 209–224. ISSN 0936-9902.
- Diedhiou, Salif; Bartsch, Peter; Kirschbaum, Frank (2007). "The embryonic and larval development of Pollimyrus isidori (Mormyridae, Osteoglossomorpha): its staging with reference to structure and behaviour" (PDF). Bulletin of Fish Biology. 9: 61–88. ISSN 1095-8649. Archived from the original (PDF) on 25 November 2015.
- Kunz, Yvette W. (2004). Developmental Biology of Teleost Fishes. Springer. p. 636. ISBN 978-14-0202-996-7.
- Bucher, Peter (2005). Zootierhaltung 5. Fische (in German). Frankfurt: Deutsch Harri GmbH. p. 890. ISBN 978-38-1711-352-1.
- Stearn, William Thomas (1959). "The Background of Linnaeus's Contributions to the Nomenclature and Methods of Systematic Biology". Systematic Zoology. 8 (1): 4–22. doi:10.2307/2411603. JSTOR 2411603.
- Linneo, Carlos (1758). Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis (in Latin). Vol. 1. Holmiae: Impensis Direct. Laurentii Salvii.
- Dayrat, Benoît (2010). "Celebrating 250 Dynamic Years of Nomenclatural Debates". Systema Naturae 250 - The Linnaean Ark (PDF). Taylor and Francis. Archived from the original (PDF) on 13 August 2011.
- Omarkhan, M. (1950). "The development of the chondrocranium of Notopterus". Journal of the Linnean Society of London, Zoology. 41 (282): 608–624. doi:10.1111/j.1096-3642.1950.tb01702.x. ISSN 1096-3642.
- Greenwood, P. Humphry; Rosen, Donn E.; et al. (1966). Phyletic Studies of Teleostean Fishes, with a Provisional Classification of Living Forms.
- Taverne, Louis (1972). "Ostéologie des genres Mormyrus Linné, Mormyrops Müller, Hyperopisus Gill, Isichthys Gill, Myomyrus Boulenger, Stomatorhinus Boulenger et Gymnarchus Cuvier. Considérations générales sur la systématique des poissons d l'ordre des Mormyriformes". Musée royal de l'Afrique centrale (in French). 200 (1–194). ISSN 0378-0953.
- Taverne, Louis (1969). "Étude Ostéologique des genres Boulengeromyrus Taverne et Géry, Genyomyrus Boulenger, Petrocephalus Marcusen (Pisces Mormyriformes)". Musee Royal de l'Afrique Centrale Annales Series IN-8 - Tervuren (in French) (174): 1–85. ISSN 0378-0953.
- Taverne, Louis (1971). "Note sur la ystématique des poissons Mormyriformes. Le problème des genres Gnathonemus Gill, Hippopotamyrus Pappenheim, Cyphomyrus Myers et les nouveaux genres Pollimyrus et Brienomyrus". Revue de Zoologie et de Botanique Africaines (in French). 84 (99–110). ISSN 0035-1814.
- Taverne, Louis (1971). "Ostéologie des genres Marcusenius Gill, Hippopotamyrus Pappenheim, Cyphomyrus Myers, Pollimyrus Taverne et Brienomyrus Taverne (Pisces, Mormyriformes)". Musée royal de l'Afrique centrale (in French). 188 (1–143). ISSN 0378-0953.
- Poll, M.; J. Gosse, P.; Orts, S. (1982). "Le genre Campylomormyrus Bleeker, 1874. Étude systématique et description d'une espèce nouvelle (Pisces, Mormyridae)". Bulletin de l'Institut Royal des Sciences Naturelles de Belgique, Biologie (in French) (5): 1-44.
- Lavoué, Sébastien; Miya, M.; et al. (2012). "Comparable Ages for the Independent Origins of Electrogenesis in African and South American Weakly Electric Fishes". PLOS ONE. 7 (5): e36287. doi:10.1371/journal.pone.0036287. PMC 3351409. PMID 22606250.
- Murray, A.M.; Cook, T.D.; et al. (2010). "A freshwater ichthyofauna from the late Eocene Birket Qarun Formation, Fayum, Egypt". Journal of Vertebrate Paleontology. 30 (3): 665–680. doi:10.1080/02724631003758060. S2CID 85047114.
- Stewart, K.M. (2001). "The freshwater fish of Neogene Africa (Miocene–Pleistocene):systematics and biogeography". Fish and Fisheries. 2 (3): 177–230. doi:10.1046/j.1467-2960.2001.00052.x.
- Stewart, Kathlyn (2009). "Fossil fish from the Nile River and its southern basins, pp. 677-704". The Nile: Origin, Environments, Limnology and Human Use. Springer Science + Business Media B. V. p. 818. ISBN 9781402097263.
- Kumazawa, Y.; Nishida, M. (2000). "Molecular phylogeny of osteoglossoids: a new model for Gondwanian origin and plate tectonic transportation of the Asian arowana" (PDF). Mol Biol Evol. 17 (12): 1869–1878. doi:10.1093/oxfordjournals.molbev.a026288. PMID 11110903.
- Inouea, Jun G.; Kumazawab, Yoshinori; et al. (2009). "The historical biogeography of the freshwater knifefishes using mitogenomic approaches: A Mesozoic origin of the Asian notopterids (Actinopterygii: Osteoglossomorpha)" (PDF). Molecular Phylogenetics and Evolution. 51 (3): 486–499. doi:10.1016/j.ympev.2009.01.020. PMID 19444960.
- Lavoué, Sébastien; et al. (2003). "Phylogenetic utility of the first two introns of the S7 ribosomal protein gene in African electric fishes (Mormyroidea: Teleostei) and congruence with other molecular markers" (PDF). Biological Journal of the Linnean Society. 78 (2): 273–292. doi:10.1046/j.1095-8312.2003.00170.x. Archived from the original (PDF) on 30 October 2014.
- Sullivan, J. P.; et al. (2000). "Molecular systematics of the African electric fishes (Mormyroidea: Teleostei) and a model for the evolution of their electric organs |publicación=Journal of Experimental Biology" (PDF). Journal of Experimental Biology. 203 (4): 665–683. doi:10.1242/jeb.203.4.665. PMID 10648209. Archived from the original (PDF) on 30 October 2014.
- Moelants, T. (2010). "Gymnarchus niloticus". IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
- Julivert, Manuel (2003). El Sáhara: Tierras, pueblos y culturas (in Spanish). Universitat de València. p. 414. ISBN 978-84-3705-798-9.
- Moelants, T. (2010). "Campylomormyrus bredoi". IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
- Departamento de Pesca y Acuicultura, FAO (2011). "B-13: Peces de agua dulce diversos. Capturas por especies, áreas de pesca y países o áreas". Anuario 2009 FAO. Estadísticas de pesca y acuicultura: Capturas (PDF) (in English, Spanish, and French). Organización de las Naciones Unidas para la Alimentación y la Agricultura.
- Moelants, T. (2010). "Ivindomyrus opdenboschi (Mormyre)". IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
- Moelants, T. (2010). "Marcusenius cuangoanus". IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
- Moelants, T. (2010). "Marcusenius brucii". IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
- Moelants, T. (2010). "Marcusenius abadii". IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
- Moelants, T. (2010). "Marcusenius furcidens". IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
- Moelants, T. (2010). "Mormyrus cyaneus". IUCN 2011. IUCN Red List of Threatened Species. Version 2011.2.
