Oxofluoxymesterone
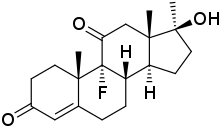
Oxofluoxymesterone (developmental code name U-6596), or ketofluoxymesterone, is an androgen and anabolic steroid (AAS) which was never marketed.[1][2][3] It was assessed in the treatment of breast cancer in women in the 1970s and showed effectiveness similar to that of other AAS.[1][2][4] The drug is the 11-dehydrogenated analogue and a metabolite of fluoxymesterone.[1][2][3][5]
 | |
| Clinical data | |
|---|---|
| Other names | U-6596; Ketofluoxymesterone; 11-Oxofluoxymesterone; 11-Ketofluoxymesterone; 9α-Fluoro-11-oxo-17α-methyltestosterone; 9α-Fluoro-17α-methylandrost-4-en-17β-ol-3,11-dione |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H27FO3 |
| Molar mass | 334.431 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- Gordan, G. S. (1976). "Cancer in Man". Anabolic-Androgenic Steroids. pp. 499–513. doi:10.1007/978-3-642-66353-6_16. ISBN 978-3-642-66355-0.
- Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 504–. ISBN 978-3-642-66353-6.
- Kammerer RC, Merdink JL, Jagels M, Catlin DH, Hui KK (1990). "Testing for fluoxymesterone (Halotestin) administration to man: identification of urinary metabolites by gas chromatography-mass spectrometry". J. Steroid Biochem. 36 (6): 659–66. doi:10.1016/0022-4731(90)90185-u. PMID 2214783.
- Dao, Thomas L. (1975). "Pharmacology and Clinical Utility of Hormones in Hormone Related Neoplasms". In Alan C. Sartorelli; David G. Johns (eds.). Antineoplastic and Immunosuppressive Agents. pp. 170–192. doi:10.1007/978-3-642-65806-8_11. ISBN 978-3-642-65806-8.
- Fürstenberger C, Vuorinen A, Da Cunha T, Kratschmar DV, Saugy M, Schuster D, Odermatt A (2012). "The anabolic androgenic steroid fluoxymesterone inhibits 11β-hydroxysteroid dehydrogenase 2-dependent glucocorticoid inactivation". Toxicol. Sci. 126 (2): 353–61. doi:10.1093/toxsci/kfs022. PMID 22273746.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.