Woodpecker
Woodpeckers are part of the bird family Picidae, which also includes the piculets, wrynecks and sapsuckers.[1] Members of this family are found worldwide, except for Australia, New Guinea, New Zealand, Madagascar and the extreme polar regions. Most species live in forests or woodland habitats, although a few species are known that live in treeless areas, such as rocky hillsides and deserts, and the Gila woodpecker specialises in exploiting cacti.
| Woodpecker Temporal range: Late Oligocene to present | |
|---|---|
 | |
| Pileated woodpecker ⓘ | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Clade: | Dinosauria |
| Class: | Aves |
| Order: | Piciformes |
| Suborder: | Pici |
| Infraorder: | Picides |
| Family: | Picidae Leach, 1820 |
| Subfamilies | |
| |
Members of this family are chiefly known for their characteristic behaviour. They mostly forage for insect prey on the trunks and branches of trees, and often communicate by drumming with their beaks, producing a reverberatory sound that can be heard at some distance. Some species vary their diet with fruits, birds' eggs, small animals, tree sap, human scraps, and carrion. They usually nest and roost in holes that they excavate in tree trunks, and their abandoned holes are of importance to other cavity-nesting birds. They sometimes come into conflict with humans when they make holes in buildings or feed on fruit crops, but perform a useful service by their removal of insect pests on trees.
The Picidae are one of nine living families in the order Piciformes, the others being barbets (comprising three families), toucans, toucan-barbets, and honeyguides, which (along with woodpeckers) comprise the clade Pici, and the jacamars and puffbirds in the clade Galbuli. DNA sequencing has confirmed the sister relationships of these two groups. The family Picidae includes about 240 species arranged in 35 genera. Almost 20 species are threatened with extinction due to loss of habitat or habitat fragmentation, with one, the Bermuda flicker, being extinct and a further two possibly being so.
General characteristics

Woodpeckers include the tiny piculets, the smallest of which appears to be the bar-breasted piculet at 7.5 cm (3.0 in) in length and a weight of 8.9 g (0.31 oz).[2][3] Some of the largest woodpeckers can be more than 50 cm (20 in) in length. The largest surviving species is the great slaty woodpecker, which weighs 430 g (15 oz) on average and up to 563 g (19.9 oz), and measures 45 to 55 cm (18 to 22 in), but the extinct imperial woodpecker, at 55 to 61 cm (22 to 24 in), and ivory-billed woodpecker, around 48 to 53 cm (19 to 21 in) and 516 g (18.2 oz), were probably both larger.[4][3][5][6][7]
The plumage of woodpeckers varies from drab to conspicuous. The colours of many species are based on olive and brown and some are pied, suggesting a need for camouflage; others are boldly patterned in black, white, and red, and many have a crest or tufted feathers on their crowns. Woodpeckers tend to be sexually dimorphic, but differences between the sexes are generally small; exceptions to this are Williamson's sapsucker and the orange-backed woodpecker, which differ markedly. The plumage is moulted fully once a year apart from the wrynecks, which have an additional partial moult before breeding.[8]
Woodpeckers, piculets, and wrynecks all possess characteristic zygodactyl feet, consisting of four toes, the first (hallux) and the fourth facing backward and the second and third facing forward. This foot arrangement is good for grasping the limbs and trunks of trees. Members of this family can walk vertically up tree trunks, which is beneficial for activities such as foraging for food or nest excavation. In addition to their strong claws and feet, woodpeckers have short, strong legs. This is typical of birds that regularly forage on trunks. Exceptions are the black-backed woodpecker and the American and Eurasian three-toed woodpeckers, which have only three toes on each foot. The tails of all woodpeckers, except the piculets and wrynecks, are stiffened, and when the bird perches on a vertical surface, the tail and feet work together to support it.[4]
Woodpeckers have strong bills that they use for drilling and drumming on trees, and long, sticky tongues for extracting food (insects and larvae).[4] Woodpecker bills are typically longer, sharper, and stronger than the bills of piculets and wrynecks, but their morphology is very similar. The bill's chisel-like tip is kept sharp by the pecking action in birds that regularly use it on wood. The beak consists of three layers; an outer sheath called rhamphotheca, made of scales formed from keratin proteins, an inner layer of bone which has a large cavity and mineralised collagen fibers, and a middle layer made of porous bone which connects the two other layers.
Furthermore, the tongue bone (or hyoid bone) of the woodpecker is very long, and winds around the skull through a special cavity, thereby cushioning the brain.[9] Combined, this anatomy helps the beak absorb mechanical stress.[10] Species of woodpecker and flicker that use their bills in soil or for probing as opposed to regular hammering tend to have longer and more decurved bills. Due to their smaller bill size, many piculets and wrynecks forage in decaying wood more often than woodpeckers. Their long, sticky tongues, which possess bristles, aid these birds in grabbing and extracting insects from deep within a hole in a tree. The tongue was reported to be used to spear grubs, but more detailed studies published in 2004 have shown that the tongue instead wraps around the prey before being pulled out.[11]
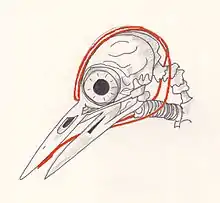
Many of the foraging, breeding, and signaling behaviors of woodpeckers involve drumming and hammering using their bills.[12] To prevent brain damage from the rapid and repeated powerful impacts, woodpeckers have a number of physical features that protect their brains.[13] These include a relatively small and smooth brain, narrow subdural space, little cerebrospinal fluid surrounding it to prevent it from moving back and forth inside the skull during pecking, the orientation of the brain within the skull (which maximises the contact area between the brain and the skull) and the short duration of contact. The skull consists of strong but compressible, sponge-like bone, which is most concentrated in the forehead and the back of the skull.[13] Another anatomical adaptation of woodpeckers is the enormously elongated hyoid bone which subdivides, passes on either side of the spinal column and wraps around the brain case, before ending in the right nostril cavity. It plays the role of safety-belt.[9]
Computer simulations have shown that 99.7% of the energy generated in pecking is stored in the form of strain energy, which is distributed throughout the bird's body, with only a small remaining fraction of the energy going into the brain. The pecking also causes the woodpecker's skull to heat up, which is part of the reason why they often peck in short bursts with brief breaks in between, giving the head some time to cool.[14] During the millisecond before contact with wood, a thickened nictitating membrane closes, protecting the eye from flying debris.[15] These membranes also prevent the retina from tearing. Their nostrils are also protected; they are often slit-like and have special feathers to cover them. Woodpeckers are capable of repeated pecking on a tree at high decelerations on the order of 10,000 m/s2 (33,000 ft/s2) (1000 g).[16]
Some large woodpeckers such as Dryocopus have a fast, direct form of flight, but the majority of species have a typical undulating flight pattern consisting of a series of rapid flaps followed by a swooping glide. Many birds in the genus Melanerpes have distinctive, rowing wing-strokes while the piculets engage in short bursts of rapid direct flight.[17]
Distribution, habitat, and movements

Global distribution
Woodpeckers have a mostly cosmopolitan distribution, although they are absent from Australasia, Madagascar, and Antarctica. They are also absent from some of the world's oceanic islands, although many insular species are found on continental islands. The true woodpeckers, subfamily Picinae, are distributed across the entire range of the family. The Picumninae piculets have a pantropical distribution, with species in Southeast Asia, Africa, and the Neotropics, with the greatest diversity being in South America.[18] The second piculet subfamily, the Sasiinae, contains the African piculet and two species in the genus Sasia that are found in Southeast Asia.[19] The wrynecks (Jynginae) are found exclusively in the Old World, with the two species occurring in Europe, Asia, and Africa.[18]
Most woodpeckers are sedentary, but a few examples of migratory species are known, such as the rufous-bellied woodpecker, yellow-bellied sapsucker,[18] and Eurasian wryneck, which breeds in Europe and west Asia and migrates to the Sahel in Africa in the winter.[20] More northerly populations of Lewis's woodpecker, northern flicker, Williamson's sapsucker, red-breasted sapsucker, and red-naped sapsucker all move southwards in the fall in North America.[18] Most woodpecker movements can be described as dispersive, such as when young birds seek territories after fledging, or eruptive, to escape harsh weather conditions. Several species are altitudinal migrants, for example the grey-capped pygmy woodpecker, which moves to lowlands from hills during winter. The woodpeckers that do migrate, do so during the day.[4]
Habitat requirements
Overall, woodpeckers are arboreal birds of wooded habitats. They reach their greatest diversity in tropical rainforests, but occur in almost all suitable habitats, including woodlands, savannahs, scrublands, and bamboo forests. Even grasslands and deserts have been colonised by various species. These habitats are more easily occupied where a small number of trees exist, or in the case of desert species like the Gila woodpecker, tall cacti are available for nesting.[21] Some are specialists and are associated with coniferous or deciduous woodlands, or even, like the acorn woodpecker, with individual tree genera (oaks in this case). Other species are generalists and are able to adapt to forest clearance by exploiting secondary growth, plantations, orchards, and parks. In general, forest-dwelling species need rotting or dead wood on which to forage.[22]
Several species are adapted to spending a portion of their time feeding on the ground, and a very small minority have abandoned trees entirely and nest in holes in the ground. The ground woodpecker is one such species, inhabiting the rocky and grassy hills of South Africa,[23] and the Andean flicker is another.[22]
The Swiss Ornithological Institute has set up a monitoring program to record breeding populations of woodland birds. This has shown that deadwood is an important habitat requirement for the black woodpecker, great spotted woodpecker, middle spotted woodpecker, lesser spotted woodpecker, European green woodpecker, and Eurasian three-toed woodpecker. Populations of all these species increased by varying amounts from 1990 to 2008. During this period, the amount of deadwood in the forest increased and the range of the white-backed woodpecker enlarged as it extended eastwards. With the exception of the green and middle-spotted woodpeckers, the increase in the amount of deadwood is likely to be the major factor explaining the population increase of these species.[24]
Behavior
Most woodpeckers live solitary lives, but their behavior ranges from highly antisocial species that are aggressive towards their own kind, to species that live in groups. Solitary species defend such feeding resources as a termite colony or fruit-laden tree, driving away other conspecifics and returning frequently until the resource is exhausted. Aggressive behaviors include bill pointing and jabbing, head shaking, wing flicking, chasing, drumming, and vocalizations. Ritual actions do not usually result in contact, and birds may "freeze" for a while before they resume their dispute. The colored patches may be flouted, and in some instances, these antagonistic behaviors resemble courtship rituals.[25]
Group-living species tend to be communal group breeders.[25] In addition to these species, a number of species may join mixed-species foraging flocks with other insectivorous birds, although they tend to stay at the edges of these groups. Joining these flocks allows woodpeckers to decrease their anti-predator vigilance and increase their feeding rate.[26] Woodpeckers are diurnal, roosting at night inside holes and crevices. In many species the roost will become the nest-site during the breeding season, but in some species they have separate functions; the grey-and-buff woodpecker makes several shallow holes for roosting which are quite distinct from its nesting site. Most birds roost alone and will oust intruders from their chosen site, but the Magellanic woodpecker and acorn woodpecker are cooperative roosters.[25]
Drumming
Drumming is a form of nonvocal communication used by most species of woodpeckers, and involves the bill being repeatedly struck on a hard surface with great rapidity. After a pause, the drum roll is repeated, with each species having a pattern that is unique in the number of beats in the roll, the length of the roll, the length of the gap between rolls, and the cadence.[27][28] The drumming is mainly a territorial call, equivalent to the song of a passerine.[29] Woodpeckers choose a surface that resonates, such as a hollow tree, and may use man-made structures such as gutters and downpipes.[30] Drumming serves for the mutual recognition of conspecifics and plays a part in courtship rituals. Individual birds are thought to be able to distinguish the drumming of their mates and those of their neighbors.[31] Drumming can be reliably used to distinguish between multiple species in a region, even if those species are phenotypically similar. Cadence (or the mean number of drum beats per second) is heavily conserved within species.[32] Comparative analyses within species between distant geographic populations have shown that cadence is heavily conserved across species' respective ranges, indicating that there likely aren't 'dialects' as seen in passerine song.[33] Drumming in woodpeckers is controlled by a set of nuclei in the forebrain that closely resemble the brain regions that underlie song learning and production in many songbirds.[34] A 2023 study revealed a strong association between extractive foraging and relative brain size across the Family Picidae, indicating that a larger brain doesn't necessarily result in more powerful drumming abilities, but is implicated in foraging behaviors, as the act of sensing and retrieving wood-boring larvae from woody substrates likely requires an increase in sensory and motor control capabilities.[35]
Calls
Woodpeckers do not have such a wide range of songs and calls as do passerine birds, and the sounds they make tend to be simpler in structure. Calls produced include brief, high-pitched notes, trills, rattles, twittering, whistling, chattering, nasal churrs, screams, and wails. These calls are used by both sexes in communication and are related to the circumstances of the occasion; these include courtship, territorial disputes, and alarm calls. Each species has its own range of calls, which tend to be in the 1.0 to 2.5 kHz range for efficient transmission through forested environments. Mated couples may exchange muted, low-pitched calls, and nestlings often issue noisy begging calls from inside their nest cavity.[29] The wrynecks have a more musical song, and in some areas, the song of the newly arrived Eurasian wryneck is considered to be the harbinger of spring.[36] The piculets either have a song consisting of a long, descending trill, or a descending series of two to six (sometimes more) individual notes, and this song alerts ornithologists to the presence of the birds, as they are easily overlooked.[37]
Diet and feeding

Most woodpecker species feed on insects and other invertebrates living under bark and in wood, but overall, the family is characterized by its dietary flexibility, with many species being both highly omnivorous and opportunistic. The diet includes ants, termites, beetles and their larvae, caterpillars, spiders, other arthropods, bird eggs, nestlings, small rodents, lizards, fruit, nuts, and sap. Many insects and their grubs are taken from living and dead trees by excavation. The bird may hear sounds from inside the timber indicating where creating a hole would be productive.[25] Crustaceans, molluscs, and carrion may be eaten by some species, including the great spotted woodpecker, and bird feeders are visited for suet and domestic scraps.[38]
Other means are also used to garner prey. Some species, such as the red-naped sapsucker, sally into the air to catch flying insects, and many species probe into crevices and under bark, or glean prey from leaves and twigs. The rufous woodpecker specialises in attacking the nests of arboreal ants, and the buff-spotted woodpecker feeds on and nests in termite mounds. Other species, such as the wrynecks and the Andean flicker, feed wholly or partly on the ground.[25]
Ecologically, woodpeckers help to keep trees healthy by keeping them from suffering mass infestations. The family is noted for its ability to acquire wood-boring grubs from the trunks and branches, whether the timber is alive or dead. Having hammered a hole into the wood, the prey is extracted by use of a long, barbed tongue. Woodpeckers consume beetles that burrow into trees, removing as many as 85% of emerald ash borer larvae from individual ash trees.[39]
The ability to excavate allows woodpeckers to obtain tree sap, an important source of food for some species. Most famously, the sapsuckers (genus Sphyrapicus) feed in this fashion, but the technique is not restricted to these, and others such as the acorn woodpecker and white-headed woodpecker also feed on sap. The technique was once thought to be restricted to the New World, but Old World species, such as the Arabian woodpecker and great spotted woodpecker, also feed in this way.[4]
Breeding

All members of the family Picidae nest in cavities, nearly always in the trunks and branches of trees, well away from the foliage. Where possible, an area of rotten wood surrounded by sound timber is used. Where trees are in short supply, the gilded flicker and ladder-backed woodpecker excavate holes in cactus, and the Andean flicker and ground woodpecker dig holes in earth banks. The campo flicker sometimes chooses termite mounds, the rufous woodpecker prefers to use ants' nests in trees and the bamboo woodpecker specialises in bamboos.[40] Woodpeckers also excavate nest holes in residential and commercial structures and wooden utility poles.[39]
Woodpeckers and piculets excavate their own nests, but wrynecks do not, and need to find pre-existing cavities. A typical nest has a round entrance hole that just fits the bird, leading to an enlarged vertical chamber below. No nesting material is used, apart from some wood chips produced during the excavation; other wood chips are liberally scattered on the ground, thus providing visual evidence of the site of the nest.[41] Many species of woodpeckers excavate one hole per breeding season, sometimes after multiple attempts. It takes around a month to finish the job and abandoned holes are used by other birds and mammals that are cavity nesters unable to excavate their own holes.[42]
Cavities are in great demand for nesting by other cavity nesters, so woodpeckers face competition for the nesting sites they excavate from the moment the hole becomes usable. This may come from other species of woodpecker, or other cavity-nesting birds such as swallows and starlings. Woodpeckers may aggressively harass potential competitors, and also use other strategies to reduce the chance of being usurped from their nesting sites; for example, the red-crowned woodpecker digs its nest in the underside of a small branch, which reduces the chance that a larger species will take it over and expand it.[43]
Members of Picidae are typically monogamous, with a few species breeding cooperatively and some polygamy reported in a few others.[44] Polyandry, where a female raises two broods with two separate males, has also been reported in the West Indian woodpecker.[45] Another unusual social system is that of the acorn woodpecker, which is a polygynandrous cooperative breeder where groups of up to 12 individuals breed and help to raise the young.[4] Young birds from previous years may stay behind to help raise the group's young, and studies have found reproductive success for the group goes up with group size, but individual success goes down. Birds may be forced to remain in groups due to a lack of habitat to which to disperse.[46]

A pair works together to help build the nest, incubate the eggs, and raise their altricial young. In most species, though, the male does most of the nest excavation and takes the night shift while incubating the eggs. A clutch usually consists of two to five round, white eggs. Since these birds are cavity nesters, their eggs do not need to be camouflaged and the white color helps the parents to see them in dim light. The eggs are incubated for about 11–14 days before they hatch. About 18–30 days are then needed before the chicks are fully fledged and ready to leave the nest. In most species, soon after this, the young are left to fend for themselves, exceptions being the various social species, and the Hispaniolan woodpecker, where adults continue to feed their young for several months. In general, cavity nesting is a successful strategy and a higher proportion of young is reared than is the case with birds that nest in the open. In Africa, several species of honeyguide are brood parasites of woodpeckers.[41]
Systematics and evolutionary history

The Picidae are just one of nine living families in the order Piciformes. Other members of this group, such as the jacamars, puffbirds, barbets, toucans, and honeyguides, have traditionally been thought to be closely related to the woodpecker family (true woodpeckers, piculets, wrynecks, and sapsuckers). The clade Pici (woodpeckers, barbets, toucans, and honeyguides) is well supported and shares a zygodactyl foot with the Galbuli (puffbirds and jacamars). More recently, several DNA sequence analyses have confirmed that Pici and Galbuli are sister groups.[47]
The name Picidae for the family was introduced by English zoologist William Elford Leach in a guide to the contents of the British Museum published in 1820.[48][49] The phylogeny has been updated according to new knowledge about convergence patterns and evolutionary history.[50][51] Most notably, the relationship of the Picinae genera has been largely clarified, and the Antillean piculet was found to be a surviving offshoot of protowoodpeckers. Genetic analysis supports the monophyly of the Picidae, which seem to have originated in the Old World, but the geographic origins of the Picinae is unclear. The Picumninae are returned as paraphyletic.[50] Morphological and behavioural characters, in addition to DNA evidence, highlights genus Hemicircus as the sister group of all remaining true woodpeckers, besides a sister-group relationship between the true woodpecker tribes Dendropicini and Malarpicini.[52]
The evolutionary history of this group is not well documented, but the known fossils allow some preliminary conclusions; the earliest known modern picids were piculet-like forms of the Late Oligocene, about 25 million years ago (Mya). By that time, however, the group was already present in the Americas and Europe, and they actually may have evolved much earlier, maybe as early as the Early Eocene (50 Mya). The modern subfamilies appear to be rather young by comparison; until the mid-Miocene (10–15 Mya), all picids seem to have been small or mid-sized birds similar to a mixture between a piculet and a wryneck. A feather enclosed in fossil amber from the Dominican Republic, dated to about 25 Mya, however, seems to indicate that the Nesoctitinae were already a distinct lineage by then.[53]
Stepwise adaptations for drilling, tapping, and climbing head first on vertical surfaces have been suggested.[52] The last common ancestor of woodpeckers (Picidae) was incapable of climbing up tree trunks or excavating nest cavities by drilling with its beak. The first adaptations for drilling (including reinforced rhamphotheca, frontal overhang, and processus dorsalis pterygoidei) evolved in the ancestral lineage of piculets and true woodpeckers. Additional adaptations for drilling and tapping (enlarged condylus lateralis of the quadrate and fused lower mandible) have evolved in the ancestral lineage of true woodpeckers (Hemicircus excepting). The inner rectrix pairs became stiffened, and the pygostyle lamina was enlarged in the ancestral lineage of true woodpeckers (Hemicircus included), which facilitated climbing head first up tree limbs. Genus Hemicircus excepting, the tail feathers were further transformed for specialized support, the pygostyle disc became greatly enlarged, and the ectropodactyl toe arrangement evolved. These latter characters may have facilitated enormous increases in body size in some lineages.[52]
Prehistoric representatives of the extant Picidae genera are treated in the genus articles. An enigmatic form based on a coracoid, found in Pliocene deposits of New Providence in the Bahamas, has been described as Bathoceleus hyphalus and probably also is a woodpecker.[54]
The following cladogram is based on the comprehensive molecular phylogenetic study of the woodpeckers published in 2017 together with the list of bird species maintained by Frank Gill, Pamela Rasmussen and David Donsker on behalf of the International Ornithological Committee (IOC). The Cuban green woodpecker in the monotypic genus Xiphidiopicus was not included in the study.[55][56] The relative positions of Picumninae, Sasiinae and Picinae in the cladogram are uncertain. In the 2017 study the results depended upon which of two different statistical procedures were used to analyse the DNA sequence data. One method found that Sasiinae was sister to Picinae (as shown below), the other method found that Sasiinae was sister to a clade containing both Picumninae and Picinae.[55]
| Picidae |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
List of genera

(Picumnus temminckii)
_female.jpg.webp)
The woodpecker family Picidae contains 37 genera.[56] For more detail, see list of woodpecker species.
_female.JPG.webp)
_female.JPG.webp)
Family: Picidae
- Subfamily: Jynginae – wrynecks
- Jynx (2 species)
- Subfamily: Picumninae – piculets[57]
- Picumnus – piculets (26 species)
- Subfamily: Sasiinae[19]
- Verreauxia – African piculet
- Sasia – Asian piculets (2 species)
- Subfamily: Picinae – true woodpeckers
- Tribe Nesoctitini
- Nesoctites – monotypic: Antillean piculet
- Tribe Hemicircini
- Hemicircus – 2 species
- Tribe Picini
- Micropternus – monotypic: rufous woodpecker
- Meiglyptes – 4 species
- Gecinulus – 3 species
- Dinopium – 5 species (flamebacks)
- Picus – 14 species
- Chrysophlegma – 3 species
- Pardipicus – 2 species
- Geocolaptes – monotypic: ground woodpecker
- Campethera – 11 species
- Mulleripicus – 4 species
- Dryocopus – 6 species
- Celeus – 13 species
- Piculus – 7 species
- Colaptes – 14 species
- Tribe Campephilini
- Campephilus – 12 species
- Blythipicus – 2 species
- Reinwardtipicus – monotypic: orange-backed woodpecker
- Chrysocolaptes – 9 species (flamebacks)
- Tribe Melanerpini
- Sphyrapicus – 4 species (sapsuckers)
- Melanerpes – 24 species
- Picoides – 3 species
- Yungipicus – 7 species
- Leiopicus – monotypic: yellow-crowned woodpecker
- Dendrocoptes – 3 species
- Chloropicus – 3 species
- Dendropicos – 12 species
- Dendrocopos – 12 species
- Dryobates – 5 species
- Leuconotopicus – 6 species
- Veniliornis – 14 species
- Xiphidiopicus – monotypic: Cuban green woodpecker
- Tribe Nesoctitini
- Incertae sedis fossils
- Genus: †Palaeopicus (Late Oligocene of France)
- †Picidae gen. et sp. indet. (Middle Miocene of New Mexico, US)
- †Picidae gen. et sp. indet. (Late Miocene of Gargano Peninsula, Italy)
- Genus: †Palaeonerpes (Ogallala Early Pliocene of Hitchcock County, US) – possibly dendropicine
- Genus: †Pliopicus (Early Pliocene of Kansas, US) – possibly dendropicine
- cf. Colaptes DMNH 1262 (Early Pliocene of Ainsworth, US) – malarpicine?
Relationship with humans
In general, humans consider woodpeckers in a favourable light; they are viewed as interesting birds and fascinating to watch as they drum or forage, but their activities are not universally appreciated.[58] Many woodpecker species are known to excavate holes in buildings, fencing, and utility poles, creating health and/or safety issues for affected structures. Such activity is very difficult to discourage and can be costly to repair.[59]
Woodpeckers also drum on various reverberatory structures on buildings such as gutters, downspouts, chimneys, vents, and aluminium sheeting.[60] Drumming is a less-forceful type of pecking that serves to establish territory and attract mates.[59] Houses with shingles or wooden boarding are also attractive as possible nesting or roosting sites, especially when close to large trees or woodland. Several exploratory holes may be made, especially at the junctions of vertical boards or at the corners of tongue-and-groove boarding. The birds may also drill holes in houses as they forage for insect larvae and pupae hidden behind the woodwork.[60]
Woodpeckers sometimes cause problems when they raid fruit crops, but their foraging activities are mostly beneficial as they control forest insect pests such as the woodboring beetles that create galleries behind the bark and can kill trees. They also eat ants, which may be tending sap-sucking pests such as mealybugs, as is the case with the rufous woodpecker in coffee plantations in India.[58] Woodpeckers can serve as indicator species, demonstrating the quality of the habitat. Their hole-making abilities make their presence in an area an important part of the ecosystem, because these cavities are used for breeding and roosting by many bird species that are unable to excavate their own holes, as well as being used by various mammals and invertebrates.[58]
The spongy bones of the woodpecker's skull and the flexibility of its beak, both of which provide protection for the brain when drumming, have provided inspiration to engineers; a black box needs to survive intact when a plane falls from the sky, and modelling the black box with regard to a woodpecker's anatomy has increased the resistance of this device to damage 60-fold.[61] The design of protective helmets is another field being influenced by the study of woodpeckers.[61]
One of the accounts of the founding of Rome, preserved in the work known as Origo Gentis Romanae, refers to a legend of a woodpecker bringing food to the boys Romulus and Remus during the time they were abandoned in the wild, thus enabling them to survive and play their part in history.
Popular culture
The Pokémon Pikipek was introduced in the seventh generation games Pokémon Sun and Moon. In addition to being a visual homage to a pileated woodpecker, entries in the game's Pokédex encyclopedia describes the small Flying-type as analogous to its real-world counterpart.[62] Its later forms (called "evolutions" in the series) Trumbeak and Toucannon resemble a honeyguide and toucan, respectively, perhaps as a tongue-in-cheek reference to the phylogenetic relationship woodpeckers share with these Piciformes families.
Status and conservation

In a global survey of the risk of extinction faced by the various bird families, woodpeckers were the only bird family to have significantly fewer species at risk than would be expected.[64]
Nevertheless, several woodpeckers are under threat as their habitats are destroyed. Being woodland birds, deforestation and clearance of land for agriculture and other purposes can reduce populations dramatically. Some species adapt to living in plantations and secondary growth, or to open countryside with forest remnants and scattered trees, but some do not. A few species have even flourished when they have adapted to man-made habitats. There are few conservation projects directed primarily at woodpeckers, but they benefit whenever their habitat is conserved.[58] The red-cockaded woodpecker has been the focus of much conservation effort in the southeastern United States, with artificial cavities being constructed in the longleaf pines they favour as nesting sites.[65]
Two species of woodpeckers in the Americas, the ivory-billed woodpecker is critically endangered and the imperial woodpecker is classified as extinct in the wild, with some authorities believing them extinct, though possible but disputed ongoing sightings of ivory-billed woodpeckers have been made in the United States[66] and a small population may survive in Cuba.[63] A critically endangered species is the Okinawa woodpecker from Japan, with a single declining population of a few hundred birds. It is threatened by deforestation, golf course, dam, and helipad construction, road building, and agricultural development.[67]
Brain impact research
Anatomy
Woodpeckers possess many sophisticated shock-absorption mechanisms that help protect them from head injury. Micro-CT scans show that plate-like spongy bones are in the skull with an uneven distribution, highly accumulated in the forehead and occiput but not in other regions.[68] Along with the long hyoid bone “safety belt” the woodpecker has uneven beak lengths which drastically reduce strains when compared to equal length.[68][69] Models have shown that pecking force is changed to strain energy and stored into the body at around 99% absorption while 1% is in the head. The head also has many factors that reduce strain to the brain and small portions of energy are dissipated into the form of heat, therefore the pecks are always intermittent.[70]
Tau protein accumulation is associated with chronic traumatic encephalopathy (CTE), and thus has been studied in sports where athletes suffer repeated concussions. Tau is important as it helps hold together and stabilize brain neurons. Woodpeckers' brains share similarities to humans with CTE showing most build-up in the frontal and temporal lobes of the brain.[71] It is not yet known whether these accumulations are pathological or the result of behavioral changes. More research is being done on the subject and the woodpecker is a suitable animal model to study.[71] The orientation of the brain within the skull increases the area of contact when pecking to reduce stress on the brain, and their small size helps, given the acceleration speeds.[72]
Mechanical properties
Straight-line trajectory was theorized to be the reason why woodpeckers do not injure themselves, since centripetal forces were the cause of concussion, but they do not always peck in straight lines, so they produce and resist centripetal forces.[68] Laboratory tests show that the woodpeckers' cranial bone produces a significantly higher Young's modulus and ultimate strength scores compared to other birds its size.[73] The cranial bone has a high bone mineral density with plate-like structures that are thick with high numbers of trabeculae that are spaced closely together which all may lead to lower deformation while pecking.
The jaw apparatus was studied, looking into its cushioning effects. When comparing the same impact to the beak and to the forehead, the forehead experiences an impact force 1.72 times that of the beak, due to the contact time being 3.25 ms in the forehead and 4.9 ms in the beak. This is impulse momentum where impulse is the integral of force over time. The quadrate bone and joints play an important role in extending impact time, which decreases impact load to brain tissue.[74]
Bio-inspired ideas
Beams
Bio-inspired honeycomb sandwich beams are inspired by the woodpecker's skull design; this beam's goal is to withstand continuous impacts without the need of replacement. The BHSB is composed of carbon fiber-reinforced plastic (CFRP), this is to mimic the high-strength beak. Next is a rubber layer core for the hyoid bone for absorbing and spreading impact, a second core layer of aluminum honeycomb that is porous and light like the woodpecker's spongey bone for impact cushioning. The final layer is the same as the first a CFRP to act as the skull bone.[75] Bio-inspired honeycomb sandwich beams when compared to conventional beams reduced area damage by 50–80% and carried 40 to 5% of the level of stresses in the bottom layer while having an impact-resistance efficiency 1.65 to 16.22 times higher.
References
- Bouglouan, Nicole. "Family Picidae - Woodpeckers, Piculets, Wrynecks". oiseaux-birds.com. Retrieved 2 December 2022.
- Winkler H, Christie DA, Bonan A (2020). "Bar-breasted Piculet (Picumnus aurifrons)". In del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds.). Birds of the World. Ithaca, NY, USA: Cornell Lab of Ornithology.
- Dunning, John B. Jr., ed. (2008). CRC Handbook of Avian Body Masses (2nd ed.). CRC Press. ISBN 978-1-4200-6444-5.
- Winkler, Hans & Christie, David A. (2002), "Family Picidae (Woodpeckers)" in del Hoyo, J.; Elliot, A. & Sargatal, J. (editors). (2002). Handbook of the Birds of the World. Volume 7: Jacamars to Woodpeckers. Lynx Edicions. ISBN 978-84-87334-37-5
- Jackson JA (2020). "Ivory-billed Woodpecker (Campephilus principalis)". In Poole AF, Gill FB (eds.). Birds of the World. Ithaca, NY, USA: Cornell Lab of Ornithology.
- Howell SN, Webb S (1995). A guide to the birds of Mexico and northern Central America. Oxford University Press.
- Styring, Alison R.; Hussin, Mohamed Zakaria bin (2004). "Foraging ecology of woodpeckers in lowland Malaysian rain forests". Journal of Tropical Ecology. 20 (5): 487–494. doi:10.1017/S0266467404001579. S2CID 83528456.
- Gorman 2014, pp. 22–23
- Wang L, Cheung JT, Pu F, Li D, Zhang M, Fan Y (2011). "Why do woodpeckers resist head impact injury: a biomechanical investigation". PLOS ONE. 6 (10): e26490. Bibcode:2011PLoSO...626490W. doi:10.1371/journal.pone.0026490. PMC 3202538. PMID 22046293.
- Helmenstine T (8 May 2014). "Woodpecker Beak Shock Absorbers". Science Notes. Retrieved 24 July 2017.
- Villard P, Cuisin J (2004). "How do woodpeckers extract grubs with their tongues? A study of the Guadeloupe woodpecker (Melanerpes herminieri) in the French Indies". Auk. 121 (2): 509–514. doi:10.1642/0004-8038(2004)121[0509:HDWEGW]2.0.CO;2. S2CID 86781719.
- Gibson L (2006). "Woodpecker pecking: how woodpeckers avoid brain injury" (PDF). Journal of Zoology. 270 (3): 462–465. doi:10.1111/j.1469-7998.2006.00166.x. hdl:1721.1/70094.
- Puiu T (23 March 2017). "Why woodpeckers don't get headaches". ZME Science. Retrieved 24 July 2017.
- Gammon K (25 August 2014). "Woodpecker Bodies Cushion Collision Impact On Bird Brains". Inside Science. Retrieved 24 July 2017.
- May PR, Fuster JM, Haber J, Hirschman A (June 1979). "Woodpecker drilling behavior. An endorsement of the rotational theory of impact brain injury". Archives of Neurology. 36 (6): 370–3. doi:10.1136/bjo.86.8.843. PMC 1771249. PMID 454236.
- Gibson LJ (2006). "Woodpecker pecking: how woodpeckers avoid brain injury". Journal of Zoology. 270 (3): 462–465. doi:10.1111/j.1469-7998.2006.00166.x. hdl:1721.1/70094.
- Gorman 2014, p. 27
- Gorman 2014, p. 15
- Sangster, G.; Gaudin, J.; Fuchs, J. (2022). "A new subfamily taxon for Sasia and Verreauxia (Picidae)". Bulletin of the British Ornithologists' Club. 142 (4): 478–479. doi:10.25226/bboc.v142i4.2022.a6. S2CID 254367038.
- Reichlin TS, Schaub M, Menz MH, Mermod M, Portner P, Arlettaz R, Jenni L (2008). "Migration patterns of Hoopoe Upupa epops and Wryneck Jynx torquilla : an analysis of European ring recoveries" (PDF). Journal of Ornithology. 150 (2): 393–400. doi:10.1007/s10336-008-0361-3. S2CID 43360238.
- Korol J, Hutto R (1984). "Factors Affecting Nest Site Location in Gila Woodpeckers" (PDF). Condor. 86 (1): 73–78. doi:10.2307/1367350. JSTOR 1367350.
- Gorman 2014, p. 18
- Short L (1971). "The evolution of terrestrial woodpeckers". American Museum Novitates (2467). hdl:2246/2675.
- Mollet P, Zbinden N, Schmid H (2009). "An increase in the population of woodpeckers and other bird species thanks to an increase in the quantities of deadwood?". FAO. Retrieved 28 March 2017.
- Gorman 2014, pp. 19–20
- Kimberly S (1984). "Information Exploitation By Downy Woodpeckers in Mixed-Species Flocks". Behaviour. 91 (4): 294–311. doi:10.1163/156853984X00128.
- Miles MC, Schuppe ER, Ligon RM, Fuxjager MJ (2018). "Macroevolutionary patterning of woodpecker drums reveals how sexual selection elaborates signals under constraint". Proceedings of the Royal Society B: Biological Sciences. 285 (1873). doi:10.1098/rspb.2017.2628. PMC 5832706. PMID 29467264.
- Miles MC, Schuppe ER, Fuxjager MJ (2020). "Selection for Rhythm as a Trigger for Recursive Evolution in the Elaborate Display System of Woodpeckers". The American Naturalist. 195 (5): 772–787. doi:10.1086/707748. PMID 32364790. S2CID 212917887.
- Gorman 2014, p. 28
- Williams Jr EH (2005). The Nature Handbook: A Guide to Observing the Great Outdoors. Oxford University Press. p. 118. ISBN 978-0-19-972075-0.
- Sarkar A (2003). Fundamentals Of Animals Behaviour. Discovery Publishing House. p. 264. ISBN 978-81-7141-742-1.
- Dodenhoff, Danielle J.; Stark, Robert D.; Johnson, Eric V. (2001-02-01). "Do Woodpecker Drums Encode Information for Species Recognition?". The Condor. 103 (1): 143–150. doi:10.1093/condor/103.1.143. ISSN 0010-5422.
- Baer, Alex (2018-01-05). "Slave to the Rhythm: Variation in the Acoustic Signaling of Picoides Woodpeckers". Theses and Dissertations.
- Schuppe ER, Cantin L, Chakraborty M, Biegler MT, Jarvis ER, Chen CC (2022). "Forebrain nuclei linked to woodpecker territorial drum displays mirror those that enable vocal learning in songbirds". PLOS Biology. 20 (9): e3001751. doi:10.1371/journal.pbio.3001751. PMC 9488818. PMID 36125990.
- Cárdenas-Posada, Ghislaine; Iwaniuk, Andrew N.; Fuxjager, Matthew J. (2023-04-01). "Extractive foraging behaviour in woodpeckers evolves in species that retain a large ancestral brain". Animal Behaviour. 198: 141–152. doi:10.1016/j.anbehav.2023.02.003. ISSN 0003-3472.
- Noel T (1841). Rymes and Roundelayes. Smith. p. 144.
- Hilty SL (2002). Birds of Venezuela. Princeton University Press. p. 464. ISBN 978-1-4008-3409-9.
- Winkler H, Christie DA, Kirwan GM (2020). del Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E (eds.). "Great Spotted Woodpecker (Dendrocopos major), version 1.0". Birds of the World. Ithaca, NY, USA: Cornell Lab of Ornithology. doi:10.2173/bow.grswoo.01. S2CID 226025386.
- Graham R (24 July 2014). "Resilient Woodpeckers hard to knock – or stop". Birds News. Archived from the original on 4 April 2016. Retrieved 24 March 2016.
- Gorman 2014, p. 20
- Gorman 2014, p. 22
- Kotaka N, Matsuoka S (2002). "Secondary users of Great Spotted Woodpecker (Dendrocopos major) nest cavities in urban and suburban forests in Sapporo City, northern Japan". Ornithological Science. 1 (2): 117–122. doi:10.2326/osj.1.117.
- Short LL (1979). "Burdens of the Picid Hole-Excavating Habit" (PDF). Wilson Bulletin. 91 (1): 16–28.
- Wiktander U, Olsson O, Nilsson SG (2000). "Parental care and social mating system in the Lesser Spotted Woodpecker Dendrocopos minor". Journal of Avian Biology. 31 (4): 447–456. doi:10.1034/j.1600-048X.2000.310003.x.
- Willimont LA, Jackson JA, Jackson BJ (1991). "Classical polyandry in the West Indian woodpecker on Abaco, Bahamas" (PDF). Wilson Bulletin. 103: 124–125.
- Koenig WD (1981). "Reproductive success, group size, and the evolution of cooperative breeding in the acorn woodpecker". The American Naturalist. 117 (4): 421–443. doi:10.1086/283726. JSTOR 2460453. S2CID 85399703.
- Johansson US, Ericson GP (2003). "Molecular support for a sister group relationship between Pici and Galbulae (Piciformes sensu Wetmore 1960)" (PDF). Journal of Avian Biology. 34 (2): 185–197. doi:10.1034/j.1600-048X.2003.03103.x.
- Leach WE (1820). "Eleventh Room". Synopsis of the Contents of the British Museum. Vol. 17 (17th ed.). London: British Museum. p. 68. Although the name of the author is not specified in the document, Leach was the keeper of zoology at the time.
- Bock WJ (1994). History and Nomenclature of Avian Family-Group Names. Bulletin of the American Museum of Natural History. Vol. Number 222. New York: American Museum of Natural History. pp. 146, 192. hdl:2246/830.
- Benz BW, Robbins MB, Peterson AT (August 2006). "Evolutionary history of woodpeckers and allies (Aves: Picidae): placing key taxa on the phylogenetic tree". Molecular Phylogenetics and Evolution. 40 (2): 389–99. doi:10.1016/j.ympev.2006.02.021. PMID 16635580.
- Moore WS, Weibel AC, Agius A (2006). "Mitochondrial DNA phylogeny of the woodpecker genus Veniliornis (Picidae, Picinae) and related genera implies convergent evolution of plumage patterns" (PDF). Biological Journal of the Linnean Society. 87 (4): 611–624. doi:10.1111/j.1095-8312.2006.00586.x.
- Manegold A, Töpfer T (2013). "The systematic position of Hemicircus and the stepwise evolution of adaptations for drilling, tapping and climbing up in true woodpeckers (Picinae, Picidae)". Journal of Zoological Systematics and Evolutionary Research. 51 (1): 72–82. doi:10.1111/jzs.12000.
- Grimaldi DA, Case GR (1995). "A feather in amber from the Upper Cretaceous of New Jersey" (PDF). American Museum Novitates (3126): 1–6.
- Cracraft J, Morony Jr JJ (1969). "A new Pliocene woodpecker, with comments on the fossil Picidae" (PDF). American Museum Novitates (2400): 1–8.
- Shakya, S.B.; Fuchs, J.; Pons, J.-M.; Sheldon, F.H. (2017). "Tapping the woodpecker tree for evolutionary insight". Molecular Phylogenetics and Evolution. 116: 182–191. doi:10.1016/j.ympev.2017.09.005. PMID 28890006.
- Gill, Frank; Donsker, David; Rasmussen, Pamela, eds. (January 2023). "Woodpeckers". IOC World Bird List Version 13.1. International Ornithologists' Union. Retrieved 19 February 2023.
- Fuchs J, Ohlson J, Ericson P, Pasquet E (2006). "Molecular phylogeny and biogeographic history of the piculets (Piciformes: Picumninae)". Journal of Avian Biology. 37 (5): 487–496. doi:10.1111/j.0908-8857.2006.03768.x.
- Gorman 2014, pp. 30–32
- "Stopping Woodpecker Damage". Joy of Birds. 22 March 2018. Retrieved 27 April 2018.
- "Three Reasons Why Woodpeckers Drill Holes on Houses". Woodpeckers. Cornell Lab of Ornithology. 2002. Retrieved 6 July 2017.
- Gregory J (2012). From Woodpeckers to... Helmets. Cherry Lake. pp. 24–26. ISBN 978-1-61080-582-7.
- "Pikipek | Pokédex | More at Pokemon.com". www.pokemon.com. Retrieved 2023-04-24.
- BirdLife International 2018 (2018) [amended version of 2016 assessment]. "Campephilus principalis". IUCN Red List of Threatened Species. 2018: e.T22681425A125486020. doi:10.2305/IUCN.UK.2016-3.RLTS.T22681425A125486020.en. Retrieved 8 January 2020.
- Purvis A, Rambaut A (June 1995). "Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data". Computer Applications in the Biosciences. 11 (3): 247–51. doi:10.1098/rspb.1997.0057. PMC 1688257. PMID 7583692.
- Copeyon CK, Walters JR, Carter III JH (1991). "Induction of Red-Cockaded Woodpecker Group Formation by Artificial Cavity Construction". The Journal of Wildlife Management. 55 (4): 549–556. doi:10.2307/3809497. JSTOR 3809497.
- "The search for the ivory-billed woodpecker". Big Woods Conservation Partnership. Retrieved 2017-03-26.
- BirdLife International (2018). "Dendrocopos noguchii". IUCN Red List of Threatened Species. 2018: e.T22681531A125513230. doi:10.2305/IUCN.UK.2018-2.RLTS.T22681531A125513230.en. Retrieved 12 November 2021.
- Wang L, Cheung JT, Pu F, Li D, Zhang M, Fan Y (2011-10-26). Briffa M (ed.). "Why do woodpeckers resist head impact injury: a biomechanical investigation". PLOS ONE. 6 (10): e26490. Bibcode:2011PLoSO...626490W. doi:10.1371/journal.pone.0026490. PMC 3202538. PMID 22046293.
- May PR, Newman P, Fuster JM, Hirschman A (February 1976). "Woodpeckers and Head Injury". The Lancet. 307 (7957): 454–455. doi:10.1016/s0140-6736(76)91477-x. PMID 55721. S2CID 28685873.
- "How the woodpecker avoids brain injury despite high-speed impacts via optimal anti-shock body structure". phys.org. 2014-08-11. Retrieved 2021-04-16.
- Farah G, Siwek D, Cummings P (2018-02-02). "Tau accumulations in the brains of woodpeckers". PLOS ONE. 13 (2): e0191526. Bibcode:2018PLoSO..1391526F. doi:10.1371/journal.pone.0191526. PMC 5796688. PMID 29394252.
- Gibson LJ (November 2006). "Woodpecker pecking: how woodpeckers avoid brain injury". Journal of Zoology. 270 (3): 462–465. doi:10.1111/j.1469-7998.2006.00166.x. hdl:1721.1/70094.
- Wang L, Zhang H, Fan Y (November 2011). "Comparative study of the mechanical properties, micro-structure, and composition of the cranial and beak bones of the great spotted woodpecker and the lark bird". Science China Life Sciences. 54 (11): 1036–41. doi:10.1007/s11427-011-4242-2. PMID 22173310. S2CID 25697639.
- Xu P, Ni Y, Lu S, Liu S, Zhou X, Fan Y (January 2021). "The cushioning function of woodpecker's jaw apparatus during the pecking process". Computer Methods in Biomechanics and Biomedical Engineering. 24 (5): 527–537. doi:10.1080/10255842.2020.1838489. PMID 33439040. S2CID 231596453.
- Abo Sabah SH, Kueh AB, Al-Fasih MY (April 2018). "Bio-inspired vs. conventional sandwich beams: A low-velocity repeated impact behavior exploration". Construction and Building Materials. 169: 193–204. doi:10.1016/j.conbuildmat.2018.02.201.
Cited sources
- Gorman G (2014). Woodpeckers of the World: A Photographic Guide. Firefly Books. ISBN 978-1-77085-309-6.
Further reading
- Dufort MJ (January 2016). "An augmented supermatrix phylogeny of the avian family Picidae reveals uncertainty deep in the family tree". Molecular Phylogenetics and Evolution. 94 (Pt A): 313–26. doi:10.1016/j.ympev.2015.08.025. PMID 26416706.
- Fuchs J, Pons JM (July 2015). "A new classification of the Pied Woodpeckers assemblage (Dendropicini, Picidae) based on a comprehensive multi-locus phylogeny". Molecular Phylogenetics and Evolution. 88: 28–37. doi:10.1016/j.ympev.2015.03.016. PMID 25818851.
- Fuchs J, Pons JM, Bowie RC (March 2017). "Biogeography and diversification dynamics of the African woodpeckers". Molecular Phylogenetics and Evolution. 108: 88–100. doi:10.1016/j.ympev.2017.01.007. PMID 28089840.
- Gorman G (2004). Woodpeckers of Europe: a study of European Picidae. Chalfont St Peter, Bucks.: Bruce Coleman. ISBN 978-1-872842-05-9.
- Gorman G (2011). The Black Woodpecker: a monograph on Dryocopus martius (1st ed.). Lynx. ISBN 978-84-96553-79-8.
- Gorman G (2020). The green woodpecker : a monograph on Picus viridis. Great Britain: Amazon/Picus Press. ISBN 9798676711870.</ref>
- Koenig WD, Haydock J (1999). "Oaks, acorns, and the geographical ecology of acorn woodpeckers". Journal of Biogeography. 26 (1): 159–165. doi:10.1046/j.1365-2699.1999.00256.x. S2CID 5068060.
- Lemaitre J, Villard MA (2005). "Foraging patterns of pileated woodpeckers in a managed Acadian forest: a resource selection function". Canadian Journal of Forest Research. 35 (10): 2387–2393. doi:10.1139/x05-148.
- Michalek KG, Winkler H (2001). "Parental care and parentage in monogamous great spotted woodpeckers (Picoides major) and middle spotted woodpeckers (Picoides medius)". Behaviour. 138 (10): 1259–1285. doi:10.1163/15685390152822210.
- Shakya SB, Fuchs J, Pons JM, Sheldon FH (November 2017). "Tapping the woodpecker tree for evolutionary insight". Molecular Phylogenetics and Evolution. 116: 182–191. doi:10.1016/j.ympev.2017.09.005. PMID 28890006.
- Stark RD, Dodenhoff DJ, Johnson EV (1998). "A quantitative analysis of woodpecker drumming" (PDF). Condor. 100 (2): 350–356. doi:10.2307/1370276. JSTOR 1370276.
- Webb DM, Moore WS (August 2005). "A phylogenetic analysis of woodpeckers and their allies using 12S, Cyt b, and COI nucleotide sequences (class Aves; order Piciformes)". Molecular Phylogenetics and Evolution. 36 (2): 233–48. doi:10.1016/j.ympev.2005.03.015. PMID 15869887.
- Wiebe KL, Swift TL (2001). "Clutch size relative to tree cavity size in northern flickers". Journal of Avian Biology. 32 (2): 167–173. doi:10.1034/j.1600-048X.2001.320210.x.
- Yom-Tov Y, Ar A (1993). "Incubation and fledging durations of woodpeckers" (PDF). Condor. 95 (2): 282–287. doi:10.2307/1369350. JSTOR 1369350.
External links
- Woodpecker videos, photos & sounds on the Internet Bird Collection
- . The American Cyclopædia. 1879.
- . . 1914.