Phosphoinositide 3-kinase inhibitor
Phosphoinositide 3-kinase inhibitors (PI3K inhibitors) are a class of medical drugs that are mainly used to treat advanced cancers. They function by inhibiting one or more of the phosphoinositide 3-kinase (PI3K) enzymes, which are part of the PI3K/AKT/mTOR pathway. This signal pathway regulates cellular functions such as growth and survival. It is strictly regulated in healthy cells, but is always active in many cancer cells, allowing the cancer cells to better survive and multiply. PI3K inhibitors block the PI3K/AKT/mTOR pathway and thus slow down cancer growth.[2][3] They are examples of a targeted therapy.[4] While PI3K inhibitors are an effective treatment, they can have very severe side effects and are therefore only used if other treatments have failed or are not suitable.[5][6]
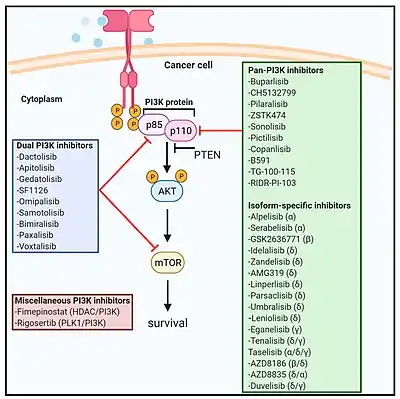
After PI3K inhibitors had been under investigation as anti-cancer drugs for several years,[7][8][9][10] the first one to be approved for treatment in clinical practice was idelalisib in 2014.[11] Several others followed, and even more are still under development (see below).[3][12]
There are different classes and isoforms of PI3Ks.[13] Class 1 PI3Ks have a catalytic subunit known as p110, with four types (isoforms) – p110 alpha (PIK3CA), p110 beta (PIK3CB), p110 gamma (PIK3CG) and p110 delta (PIK3CD).[14] All PI3K inhibitors that are currently approved inhibit one or more p110 isoforms of the class I PI3Ks. Inhibiting different p110 isoforms can have different effects,[15] e.g. PTEN-negative tumors may be more sensitive to PIK3CB inhibitors.[15]
PI3K inhibitors are also under investigation as treatments for inflammatory respiratory disease,[13][16] and are used to investigate the role of the PI3K pathway in aging.[17]
Approved for treatment
- Idelalisib (trade name Zydelig; codenamed CAL-101, GS-1101; PIK3CD inhibitor): FDA-approved in July 2014 for treatment of three types of blood cancers: treatment of relapsed or refractory chronic lymphocytic leukemia (CLL) in combination with rituximab, treatment of relapsed small lymphocytic lymphoma after at least two prior systemic therapies, and treatment of follicular lymphoma (FL) after at least two prior systemic therapies.[11]
- Copanlisib (trade name Aliqopa; codenamed BAY 80-6946; predominantly a PIK3CA and PIK3CD inhibitor): FDA-approved in September 2017 for treatment of relapsed follicular lymphoma after at least two prior systemic therapies.[18]
- Duvelisib (trade name Copiktra; codenamed INK1197, IPI-145; PIK3CD and PIK3CG inhibitor): FDA-approved on 24 September 2018 for treatment of relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) after at least two prior therapies, and treatment of relapsed or refractory follicular lymphoma after at least two prior systemic therapies.[19]
- Alpelisib (trade names Piqray and Pivikto; codenamed BYL719; PIK3CA inhibitor): FDA-approved in May 2019 for treatment of HR-positive and HER2/neu-negative breast cancer in combination with the endocrine therapy fulvestrant.[20]
- Umbralisib (trade name Ukoniq; codenamed TGR-1202, Rp-5264; PIK3CD and casein kinase CSNK1E inhibitor): FDA-approved in February 2021 for treatment of relapsed or refractory marginal zone lymphoma (MZL) after at least one prior anti-CD20-based regimen, and treatment of relapsed or refractory follicular lymphoma after at least three prior lines of systemic therapy.[21][22] As of May 31, 2022, umbralisib was withdrawn from the US market due to the decrement in overall survival and increased serious adverse events when using umbralisib.[23]
- Leniolisib (codenamed CDZ173; PIK3CD inhibitor, trade name Joenja) was tested as a potential treatment for activated PI3K delta syndrome (APDS) / PASLI disease in a placebo-controlled phase II/III trial (NCT02435173).[24][25] The trial was completed in August 2021 and results have become available in March 2022.[24] Another phase II/III trial for APDS/PASLI that serves as an extension study (NCT02859727) is still ongoing and results are expected for October 2026.[26] The FDA has approved leniolisib on March 24th 2023.
Under clinical development
Late stage
- Buparlisib (codenamed BKM120, NVP-BKM120; pan-class I PI3K inhibitor):
- The phase III trial BURAN compares buparlisib + paclitaxel to paclitaxel alone in patients with head and neck squamous cell carcinoma (HNSCC). Results are expected for December 2022.[27]
- The phase III trials BELLE-2[28][29] and BELLE-3[30][31] comparing buparlisib + fulvestrant with fulvestrant alone in patients with breast cancer both showed excessive side effects. The phase II/III trial BELLE-4 comparing buparlisib + paclitaxel with paclitaxel alone in patients with breast cancer did not improve progression-free survival and was stopped for futility at the end of phase II.[32][33] These results led the sponsor, Novartis, to cancel their breast cancer study program with buparlisib.
- Copanlisib (codenamed BAY 80-6946; predominantly a PIK3CA and PIK3CD inhibitor) ist currently undergoing three phase III trials, all of which are testing it in patients with indolent non-Hodgkin lymphoma (iNHL):
- The trial CHRONOS-2 was planned as a placebo-controlled randomized phase III trial with about 190 patients. However, recruitment was stopped after 25 patients were included and the trial continues as a non-randomized single-arm trial. Results are expected for November 2022.[34]
- The phase III trial CHRONOS-3 compares copanlisib + rituximab with placebo + rituximab in patients with relapsed iNHL. Study completion is expected for January 2023.[35] Preliminary results show a strong and significant improvement of progression-free survival under copanlisib treatment, but also considerably more severe and serious side effects.[36]
- The phase III trial CHRONOS-4 compares copanlisib + immunochemotherapy (R-CHOP regimen) with placebo + immunochemotherapy in patients with relapsed iNHL who have received 1–3 previous lines of therapy. Results are expected for February 2023.[37]
- Dactolisib (codenamed BEZ235, NVP-BEZ235; dual pan-class I PI3K and mTOR inhibitor)[38] was tested in the placebo-controlled phase III trial PROTECTOR 1 (RTB-101-204) to prevent clinically symptomatic respiratory illness in generally healthy elderly people.[39] However, the trial did not meet this endpoint.[40] Consequently, the related phase III trial PROTECTOR 2 (RTB-101-205) was terminated by the sponsor.[41] Dactolisib has also undergone several phase II trials as a potential treatment for solid tumours as well as for respiratory diseases, most of which have been terminated as of 2022.[42]
- Duvelisib (codenamed INK1197, IPI-145; PIK3CD and PIK3CG inhibitor):
- The results of the completed pivotal phase III trial DUO comparing duvelisib monotherapy with ofatumumab led to its approval for CLL/SLL[43] An extension trial to DUO was completed in 2020, but its results have not yet been published.[44]
- The phase III trial BRAVURA comparing duvelisib + rituximab + bendamustine with rituximab + bendamustine in patients with non-Hodgkin lymphoma was withdrawn by the sponsor when it was no longer expected to lead to approval.[45]
- Similarly, the phase III trial DYNAMO + R comparing duvelisib + rituximab with rituximab alone in patients with follicular lymphoma was terminated by the sponsor when it was no longer expected to lead to approval.[46]
- Idelalisib has undergone eleven phase III clinical trials as of March 2022.[47] These include the pivotal trial GS-US-312-0116 that lead to approval of idelalisib by FDA and EMA for tratment of patients with CLL. All other phase III trials testing idelalisib-based therapy as an experimental treatment, e.g. in first-line CLL and second-line NHL, had been terminated by end of 2016, mainly due to increased toxicity and mortality.[48] Two trials comparing new experimental treatments to idelalisib as a comparator and a dose optimization study in FL are still ongoing.[49][50][51]
- Parsaclisib (codenamed INCB050465, INCB 50465; PIK3CD inhibitor) will be tested as a potential treatment for different diseases in five phase III trials:
- Follicular lymphoma (FL) and marginal zone lymphoma (MZL) in the phase III trial CITADEL-302[52]
- Mantle cell lymphoma (MCL) in the phase III trial CITADEL-310[53]
- Myelofibrosis in the phase III trials LIMBER-304[54] and LIMBER-313[55]
- Warm antibody autoimmune hemolytic anemia (WAIHA) in the phase III trial PATHWAY[56]
- Paxalisib (codenamed GDC-0084; pan-class I PI3K and mTOR inhibitor) will be tested as a potential treatment for glioblastoma in the phase II/III trial GBM AGILE. The trial is currently recruiting patients as of March 2022 and will compare multiple experimental treatments including paxalisib with temozolomide + radiotherapy (+ lomustine).[57]
- Taselisib (codenamed GDC-0032, RG7604; PIK3CA inhibitor): Development was discontinued due to strong side effects and only a minor survival benefit in the phase III trial SANDPIPER in patients with breast cancer.[58][59]
- Zandelisib (codenamed ME-401; PIK3CD inhibitor) will be tested as a potential treatment for iNHL in the phase III trial COASTAL. The trial is currently recruiting patients as of March 2022 and will compare zandelisib + rituximab to chemotherapy (CHOP regimen) + rituximab. Results are expected for April 2026.[60]
- Inavolisib (codenamed GDC-0077; PIK3CA inhibitor) will be tested as a potential treatment for PIK3CA-mutant breast cancer in a phase II/III trial (NCT04191499). The trial is currently recruiting patients as of March 2022 and will compare inavolisib + palbociclib + fulvestrant with placebo + palbociclib + fulvestrant. Results are expected for September 2025.[61]
- Apitolisib (codenamed GDC-0980, GNE 390, RG7422; pan-class I PI3K and mTOR inhibitor) has undergone four phase II trials as a potential treatment for different solid tumours, three of which have been completed or terminated as of March 2022.[62]
- Bimiralisib (codenamed PQR309; brain-permeant dual PI3K/mTOR inhibitor) has undergone several phase II trials as a potential treatment for different solid tumours, all of which have been completed or terminated as of March 2022.[63]
- Eganelisib (codenamed IPI-549; PIK3CD inhibitor) is currently undergoing three phase II trials as a potential treatment for different solid tumours, with no published results as of March 2022.[64]
- Fimepinostat (codenamed CUDC-907; PI3K p110 and HDAC inhibitor): A phase II trial in patients with diffuse large B-cell lymphoma (DLBCL) was completed in 2019 but its results have not yet been published.[65] Other phase II trials with fimepinostat have been terminated.[66]
- Gedatolisib (codenamed PF-05212384, PKI-587; PIK3CA, PIK3CG and mTOR inhibitor) has undergone several phase II trials as a potential treatment for different cancers, most of which have been terminated for different reasons. As of March 2022, two phase II trials on breast cancer are still recruiting patients.[67]
- Linperlisib (codenamed YY-20394; PIK3CD inhibitor) will be tested as a potential treatment for different types of lymphoma in several phase II trials that are currently recruiting or scheduled to recruit patients as of March 2022.[68]
- Nemiralisib (codenamed GSK2269557; PIK3CD inhibitor) has undergone several phase II trials as a potential treatment for different respiratory diseases (asthma and COPD) as well as for APDS/PASLI, all of which have been completed or terminated as of March 2022. Results are available for all of these trials.[69]
- Pictilisib (codenamed GDC-0941; pan-class I PI3K inhibitor)[70] has undergone five phase II trials as a potential treatment for different solid tumours, with no results published as of March 2022.[71]
- Pilaralisib (codenamed SAR245408 and XL147; inhibitor of PIK3CA, PIK3CD, and PIK3CG) has undergone several phase II trials as a potential treatment for different solid tumours, all of which have been completed as of 2022.[72]
- Samotolisib (codenamed GTPL8918, LY3023414; triple pan-class I PI3K, mTOR, and DNA-PK inhibitor) has undergone several phase II trials as a potential treatment for different cancers, three of which have been completed or terminated and have results as of March 2022.[73]
- Seletalisib (codenamed UCB-5857; PIK3CD inhibitor) has undergone one phase II trial as a potential treatment for Sjögren syndrome. The trial has been terminated due to enrolment challenges.[74]
- Serabelisib (codenamed MLN1117 and TAK-117; PIK3CA inhibitor) is undergoing several phase II trials as a potential treatment for different cancers. As of March 2022, results have been reported for renal cell carcinoma and endometrial cancer.[75][76]
- Sonolisib (codenamed PX-866; a wortmannin derivative)[77] has undergone several phase II trials as a potential treatment for different solid tumours, all of which have been completed or terminated as of 2022.[78]
- Tenalisib (codenamed RP6530; dual PIK3CD and PIK3CG inhibitor) is undergoing several phase II trials as a potential treatment for different cancers. Two single-arm trials (in CLL and iNHL) have reported results.[79][80]
- Voxtalisib (codenamed SAR245409, XL765; pan-class I PI3K inhibitor and weaker inhibitor of mTOR), in trial for B-cell lymphomas, e.g. CLL and follicular lymphoma.[81][82]
- AMG 319 (PIK3CD inhibitor) has undergone a phase II trial as a potential treatment for HNSCC. The trial was terminated in 2018 due to safety reasons.[83]
- AZD8186 (PIK3CB and PIK3CD inhibitor) will be tested as a potential treatment for gastric cancer in a phase II trial that is currently recruiting patients as of March 2022.[84]
- GSK2636771 (PIK3CB inhibitor) has undergone several phase II trials as a potential treatment for different cancers, one of which have been completed, with no results published as of March 2022.[85]
- SF1126 is a peptidic prodrug targeting integrin receptors that converts to LY294002, one of the most widely studied dual PI3K/mTOR inhibitors.[86] A phase II trial with SF1126 has been terminated due to slow enrolment.[87]
Early stage
In early stage clinical trials[9]
- Acalisib (codenamed CAL-120, GS-9820) has completed one phase I trial in 2016. No data have been published for this trial and no further trials have been conducted since then as of March 2022.[88]
- Omipalisib (codenamed GSK2126458, GSK458) has completed two phase I trials in 2015 and 2016, respectively. No data have been published for these trials and no further trials have been conducted since then as of March 2022.[89]
- AZD8835 (PIK3CA and PIK3CD inhibitor) has completed one phase I trial in 2016. No data have been published for this trial and no further trials have been conducted since then as of March 2022.[90]
- CAL263 (PIK3CD inhibitor)[91]
- GSK1059615 (dual pan-class I PI3K and mTOR inhibitor): The phase I trial of this drug was terminated due to lack of sufficient exposure following single- and repeat-dosing.[92]
- MEN1611 (CH5132799, PA799; mainly a PIK3CA inhibitor) will be tested in a phase I/II trial with PIK3CA-mutated colorectal cancer patients that is currently recruiting patients as of March 2022.[93]
- PWT33597 (dual PIK3CA and mTOR inhibitor)[94]
- TG100-115 (mainly a PIK3CD and PIK3CG inhibitor)[95]
- ZSTK474 (mainly a PIK3CD inhibitor)[96]
Not in clinical trials
- AEZS-136 (dual PI3K and ERK inhibitor)[97]
- B591 (pan-class I PI3K inhibitor)[98]
- GNE-477 (dual PIK3CA and mTOR inhibitor with IC50 values of 4 nM and 21 nM)[99]
- Hibiscone C (irreversible PI3K inhibitor)
- IC87114 (mainly a PIK3CD inhibitor)[16]
- LY294002 (reversible PI3K inhibitor),[16] mainly used as a research tool
- PI-103 (triple pan-class I PI3K, mTOR, and DNA-PK inhibitor)[100]
- Wortmannin (irreversible PI3K inhibitor), mainly used as a research tool
See also
- PI3K/AKT/mTOR pathway for inhibitors of AKT and mTOR downstream from PI3K
- P110δ#Pharmacology, P110δ (PI3K-delta) as a drug target
References
- Mishra R, Patel H, Alanazi S, Kilroy MK, Garrett JT (March 2021). "PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects". International Journal of Molecular Sciences. 22 (7): 3464. doi:10.3390/ijms22073464. PMC 8037248. PMID 33801659.
- Hoxhaj G, Manning BD (February 2020). "The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism". Nature Reviews. Cancer. 20 (2): 74–88. doi:10.1038/s41568-019-0216-7. PMC 7314312. PMID 31686003.
- Sabbah DA, Hajjo R, Bardaweel SK, Zhong HA (October 2021). "Phosphatidylinositol 3-kinase (PI3K) inhibitors: a recent update on inhibitor design and clinical trials (2016-2020)". Expert Opinion on Therapeutic Patents. 31 (10): 877–892. doi:10.1080/13543776.2021.1924150. PMID 33970742. S2CID 234360275.
- Zhong, Lei; Li, Yueshan; Xiong, Liang; Wang, Wenjing; Wu, Ming; Yuan, Ting; Yang, Wei; Tian, Chenyu; Miao, Zhuang; Wang, Tianqi; Yang, Shengyong (2021). "Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives". Signal Transduction and Targeted Therapy. 6 (1): 201. doi:10.1038/s41392-021-00572-w. PMC 8165101. PMID 34054126.
- Curigliano G, Shah RR (February 2019). "Safety and Tolerability of Phosphatidylinositol-3-Kinase (PI3K) Inhibitors in Oncology". Drug Safety. 42 (2): 247–262. doi:10.1007/s40264-018-0778-4. PMID 30649751. S2CID 58657824.
- Hanlon A, Brander DM (December 2020). "Managing toxicities of phosphatidylinositol-3-kinase (PI3K) inhibitors". Hematology. American Society of Hematology. Education Program. 2020 (1): 346–356. doi:10.1182/hematology.2020000119. PMC 7727518. PMID 33275709.
- Flanagan (Dec 2008). "Zeroing in on PI3K Pathway". Archived from the original on 2013-01-24.
- Wu P, Liu T, Hu Y (2009). "PI3K inhibitors for cancer therapy: what has been achieved so far?". Current Medicinal Chemistry. 16 (8): 916–930. doi:10.2174/092986709787581905. PMID 19275602.
- Maira SM, Stauffer F, Schnell C, García-Echeverría C (February 2009). "PI3K inhibitors for cancer treatment: where do we stand?". Biochemical Society Transactions. 37 (Pt 1): 265–272. doi:10.1042/BST0370265. PMID 19143644.
- Heavey S, O'Byrne KJ, Gately K (April 2014). "Strategies for co-targeting the PI3K/AKT/mTOR pathway in NSCLC". Cancer Treatment Reviews. 40 (3): 445–456. doi:10.1016/j.ctrv.2013.08.006. PMID 24055012.
- "FDA approves Zydelig for three types of blood cancers". US Food and Drug Administration. July 23, 2014.
- Vanhaesebroeck B, Perry MW, Brown JR, André F, Okkenhaug K (October 2021). "PI3K inhibitors are finally coming of age". Nature Reviews. Drug Discovery. 20 (10): 741–769. doi:10.1038/s41573-021-00209-1. PMC 9297732. PMID 34127844. S2CID 235437841.
- Ito K, Caramori G, Adcock IM (April 2007). "Therapeutic potential of phosphatidylinositol 3-kinase inhibitors in inflammatory respiratory disease". The Journal of Pharmacology and Experimental Therapeutics. 321 (1): 1–8. doi:10.1124/jpet.106.111674. PMID 17021257. S2CID 1906947.
- Study results provide rationale for use of PI3K inhibitors in therapeutic settings. News-medical.net. Retrieved on 2010-11-05.
- Edgar KA, Wallin JJ, Berry M, Lee LB, Prior WW, Sampath D, et al. (February 2010). "Isoform-specific phosphoinositide 3-kinase inhibitors exert distinct effects in solid tumors". Cancer Research. 70 (3): 1164–1172. doi:10.1158/0008-5472.CAN-09-2525. PMID 20103642.
- Crabbe T (April 2007). "Exploring the potential of PI3K inhibitors for inflammation and cancer". Biochemical Society Transactions. 35 (Pt 2): 253–256. doi:10.1042/BST0350253. PMID 17371252.
- Blagosklonny MV. Anti-aging: senolytics or gerostatics (unconventional view). Oncotarget. 2021 Aug 31;12(18):1821-1835. doi:10.18632/oncotarget.28049 PMID 34504654
- "FDA approves new treatment for adults with relapsed follicular lymphoma". US Food and Drug Administration. September 14, 2017.
- "FDA Approval for duvelisib (COPIKTRA, Verastem, Inc.) for adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL)". US Food and Drug Administration. September 24, 2018.
- "FDA approves first PI3K inhibitor for breast cancer". Food and Drug Administration. 2019-05-24.
- "Ukoniq (umbralisib) tablets, for oral use" (PDF). TG Therapeutics.
- "FDA grants accelerated approval to umbralisib for marginal zone lymphoma and follicular lymphoma". U.S. Food and Drug Administration (FDA). 5 February 2021. Retrieved 5 February 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Federal Register :: Request Access". unblock.federalregister.gov. Retrieved 2022-10-06.
- Clinical trial number NCT02435173 for "Study of Efficacy of CDZ173 in Patients With APDS/PASLI" at ClinicalTrials.gov
- Rao VK, Webster S, Dalm VA, Šedivá A, van Hagen PM, Holland S, et al. (November 2017). "Effective "activated PI3Kδ syndrome"-targeted therapy with the PI3Kδ inhibitor leniolisib". Blood. 130 (21): 2307–2316. doi:10.1182/blood-2017-08-801191. PMC 5701526. PMID 28972011.
- Clinical trial number NCT02859727 for "Extension to the Study of Efficacy of CDZ173 in Patients With APDS/PASLI" at ClinicalTrials.gov
- Clinical trial number NCT04338399 for "The BURAN Study of Buparlisib in Patients With Recurrent or Metastatic HNSCC (BURAN)" at ClinicalTrials.gov
- Baselga J, Im SA, Iwata H, Cortés J, De Laurentiis M, Jiang Z, et al. (July 2017). "Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial". The Lancet. Oncology. 18 (7): 904–916. doi:10.1016/S1470-2045(17)30376-5. PMC 5549667. PMID 28576675.
- Clinical trial number NCT01610284 for "Phase III Study of BKM120/Placebo With Fulvestrant in Postmenopausal Patients With Hormone Receptor Positive HER2-negative Locally Advanced or Metastatic Breast Cancer Refractory to Aromatase Inhibitor (BELLE-2)" at ClinicalTrials.gov
- Di Leo A, Johnston S, Lee KS, Ciruelos E, Lønning PE, Janni W, et al. (January 2018). "Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): a randomised, double-blind, placebo-controlled, phase 3 trial". The Lancet. Oncology. 19 (1): 87–100. doi:10.1016/S1470-2045(17)30688-5. PMID 29223745.
- Clinical trial number NCT01633060 for "A Phase III Study of BKM120 With Fulvestrant in Patients With HR+,HER2-, AI Treated, Locally Advanced or Metastatic Breast Cancer Who Progressed on or After mTORi (BELLE-3)" at ClinicalTrials.gov
- Martín M, Chan A, Dirix L, O'Shaughnessy J, Hegg R, Manikhas A, et al. (February 2017). "A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4)". Annals of Oncology. 28 (2): 313–320. doi:10.1093/annonc/mdw562. PMID 27803006.
- Clinical trial number NCT01572727 for "A Study of the Experimental Drug BKM120 With Paclitaxel in Patients With HER2 Negative, Locally Advanced or Metastatic Breast Cancer, With or Without PI3K Activation (BELLE-4)" at ClinicalTrials.gov
- Clinical trial number NCT02369016 for "Phase III Copanlisib in Rituximab-refractory iNHL (CHRONOS-2)" at ClinicalTrials.gov
- Clinical trial number NCT02367040 for "Copanlisib and Rituximab in Relapsed Indolent B-cell Non-Hodgkin's Lymphoma (iNHL) (CHRONOS-3)" at ClinicalTrials.gov
- Matasar MJ, Capra M, Özcan M, Lv F, Li W, Yañez E, et al. (May 2021). "Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial". The Lancet. Oncology. 22 (5): 678–689. doi:10.1016/S1470-2045(21)00145-5. PMID 33848462. S2CID 233234876.
- Clinical trial number NCT02626455 for "Study of Copanlisib in Combination With Standard Immunochemotherapy in Relapsed Indolent Non-Hodgkin's Lymphoma (iNHL) (CHRONOS-4)" at ClinicalTrials.gov
- Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, Maira SM, et al. (August 2009). "NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas". Molecular Cancer Therapeutics. 8 (8): 2204–2210. doi:10.1158/1535-7163.MCT-09-0160. PMC 2752877. PMID 19671762.
- Clinical trial number NCT04668352 for "A Phase 3 Study to Determine if RTB101 Prevents Clinically Symptomatic Respiratory Illness in the Elderly" at ClinicalTrials.gov
- Kaeberlein M (2020). "RTB101 and immune function in the elderly: Interpreting an unsuccessful clinical trial". Translational Medicine of Aging. 4: 32–34. doi:10.1016/j.tma.2020.01.002. S2CID 213379077.
- Clinical trial number NCT04139915 for "Effect of RTB101 on Illness Associated With Respiratory Tract Infections in the Elderly" at ClinicalTrials.gov
- "Search for phase 2 clinical trials with Dactolisib on clinicaltrials.gov". Retrieved 2022-03-04.
- Clinical trial number NCT02004522 for "A Phase 3 Study of Duvelisib Versus Ofatumumab in Patients With Relapsed or Refractory CLL/SLL (DUO)" at ClinicalTrials.gov
- Clinical trial number NCT02049515 for "A Phase 3 Extension Study of Duvelisib and Ofatumumab in Patients With CLL/SLL Previously Enrolled in Study IPI-145-07" at ClinicalTrials.gov
- Clinical trial number NCT02576275 for "A Study of Duvelisib in Combination With Rituximab and Bendamustine vs Placebo in Combination With Rituximab and Bendamustine in Subjects With Previously-Treated Indolent Non-Hodgkin Lymphoma (BRAVURA)" at ClinicalTrials.gov
- Clinical trial number NCT02204982 for "Study of Duvelisib in Combination With Rituximab vs Rituximab in Subjects With Previously Treated Follicular Lymphoma (DYNAMO + R)" at ClinicalTrials.gov
- "Search for phase 3 clinical trials with CAL-101 on clinicaltrials.gov". Retrieved 2022-03-21.
- "Zydelig : EPAR - Scientific conclusions" (PDF). 2016-11-21.
{{cite journal}}: Cite journal requires|journal=(help) - Clinical trial number NCT02970318 for "A Study of Acalabrutinib vs Investigator's Choice of Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in R/R CLL" at ClinicalTrials.gov
- Clinical trial number NCT04666038 for "Study of LOXO-305 Versus Investigator's Choice (IdelaR or BR) in Patients With Previously Treated Chronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) (BRUIN CLL-321)" at ClinicalTrials.gov
- Clinical trial number NCT02536300 for "Dose Optimization Study of Idelalisib in Follicular Lymphoma" at ClinicalTrials.gov
- Clinical trial number NCT04796922 for "To Evaluate Efficacy and Safety of Parsaclisib Plus Either Rituximab or Obinutuzumab in R/R Follicular Lymphoma (FL) and Marginal Zone Lymphoma (MZL) (CITADEL-302)" at ClinicalTrials.gov
- Clinical trial number NCT04849715 for "A Study of Parsaclisib, a PI3Kδ Inhibitor, in Combination With Bendamustine and Rituximab in Patients With Newly Diagnosed Mantle Cell Lymphoma (CITADEL-310)" at ClinicalTrials.gov
- Clinical trial number NCT04551053 for "To Evaluate Efficacy and Safety of Parsaclisib and Ruxolitinib in Participants With Myelofibrosis Who Have Suboptimal Response to Ruxolitinib (LIMBER-304)" at ClinicalTrials.gov
- Clinical trial number NCT04551066 for "To Evaluate the Efficacy and Safety of Parsaclisib and Ruxolitinib in Participants With Myelofibrosis (LIMBER-313)" at ClinicalTrials.gov
- Clinical trial number NCT05073458 for "Study of the Efficacy and Safety of Parsaclisib in Participants With Primary Warm Autoimmune Hemolytic Anemia (PATHWAY)" at ClinicalTrials.gov
- Clinical trial number NCT03970447 for "A Trial to Evaluate Multiple Regimens in Newly Diagnosed and Recurrent Glioblastoma (GBM AGILE)" at ClinicalTrials.gov
- "Roche dumps its PhIII PI3K effort on taselisib after researchers track poor survival edge, harsh side effects for breast cancer".
- Clinical trial number NCT02340221 for "A Study of Taselisib + Fulvestrant Versus Placebo + Fulvestrant in Participants With Advanced or Metastatic Breast Cancer Who Have Disease Recurrence or Progression During or After Aromatase Inhibitor Therapy (SANDPIPER)" at ClinicalTrials.gov
- Clinical trial number NCT04745832 for "Phase 3 Study of Zandelisib (ME-401) in Combination With Rituximab in Patients With iNHL - (COASTAL)" at ClinicalTrials.gov
- Clinical trial number NCT04191499 for "A Study Evaluating the Efficacy and Safety of Inavolisib + Palbociclib + Fulvestrant vs Placebo + Palbociclib + Fulvestrant in Patients With PIK3CA-Mutant, Hormone Receptor-Positive, Her2-Negative, Locally Advanced or Metastatic Breast Cancer" at ClinicalTrials.gov
- "Search for phase 2 clinical trials with GDC-0980 on clinicaltrials.gov". Retrieved 2022-03-17.
- "Search for phase 2 clinical trials with PQR309 on clinicaltrials.gov". Retrieved 2022-03-17.
- "Search for phase 2 clinical trials with IPI-549 on clinicaltrials.gov". Retrieved 2022-03-07.
- Clinical trial number NCT02674750 for "Study to Evaluate the Efficacy and Safety of CUDC-907 in Patients With RR DLBCL, Including Patients With MYC Alterations" at ClinicalTrials.gov
- "Search for phase 2 clinical trials with CUDC-907 on clinicaltrials.gov". Retrieved 2022-03-04.
- "Search for phase 2 clinical trials with PKI-587 on clinicaltrials.gov". Retrieved 2022-03-17.
- "Search for phase 2 clinical trials with YY-20394 on clinicaltrials.gov". Retrieved 2022-03-18.
- "Search for phase 2 clinical trials with GSK2269557 on clinicaltrials.gov". Retrieved 2022-03-17.
- Sarker D, Ang JE, Baird R, Kristeleit R, Shah K, Moreno V, et al. (January 2015). "First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors". Clinical Cancer Research. 21 (1): 77–86. doi:10.1158/1078-0432.CCR-14-0947. PMC 4287394. PMID 25370471.
- "Search for phase 2 clinical trials with IPI-549 on clinicaltrials.gov". Retrieved 2022-03-07.
- "Search for phase 2 clinical trials with SAR245408 on clinicaltrials.gov". Retrieved 2022-03-09.
- "Search for phase 2 clinical trials with LY3023414 on clinicaltrials.gov". Retrieved 2022-03-17.
- Clinical trial number NCT02610543 for "UCB Proof of Concept Study in Patients With Primary Sjögren's Syndrome" at ClinicalTrials.gov
- Clinical trial number NCT02724020 for "MLN0128 and MLN0128 + MLN1117 Compared With Everolimus in the Treatment of Adults With Advanced or Metastatic Clear-Cell Renal Cell Carcinoma" at ClinicalTrials.gov
- Clinical trial number NCT02725268 for "A Study of Sapanisertib, Combination of Sapanisertib With MLN1117, Paclitaxel and Combination of Sapanisertib With Paclitaxel in Women With Endometrial Cancer" at ClinicalTrials.gov
- Howes AL, Chiang GG, Lang ES, Ho CB, Powis G, Vuori K, Abraham RT (September 2007). "The phosphatidylinositol 3-kinase inhibitor, PX-866, is a potent inhibitor of cancer cell motility and growth in three-dimensional cultures". Molecular Cancer Therapeutics. 6 (9): 2505–2514. doi:10.1158/1535-7163.MCT-06-0698. PMID 17766839.
- "Search for phase 2 clinical trials with PX-866 on clinicaltrials.gov". Retrieved 2022-03-04.
- Clinical trial number NCT04204057 for "Efficacy and Safety of Tenalisib (RP6530) in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia (CLL)" at ClinicalTrials.gov
- Clinical trial number NCT03711578 for "Efficacy and Safety Study of Tenalisib (RP6530), a Novel PI3K δ/γ Dual Inhibitor in Patients With Relapsed/Refractory Indolent Non-Hodgkin's Lymphoma (iNHL)" at ClinicalTrials.gov
- "In Focus: Voxtalisib for CLL and B-Cell Lymphomas". Cancer Therapy Advisor. March 27, 2018.
- Clinical trial number NCT01403636 for "A Study of Investigational SAR245409 in Patients With Certain Lymphoma or Leukemia" at ClinicalTrials.gov
- Clinical trial number NCT02540928 for "AMG 319 in HPV Positive and Negative HNSCC" at ClinicalTrials.gov
- Clinical trial number NCT04001569 for "AZD8186 and Paclitaxel in Advanced Gastric Cancer" at ClinicalTrials.gov
- "Search for phase 2 clinical trials with GSK2636771 on clinicaltrials.gov". Retrieved 2022-03-17.
- Garlich JR, Becker MD, Shelton CF, Qi W, Liu X, Cooke L, Mahadevan D (2010). "Phase I Study of Novel Prodrug Dual PI3K/MTOR Inhibitor SF1126 in B-Cell Malignancies". Blood. 116 (21): 1783. doi:10.1182/blood.V116.21.1783.1783.
- Clinical trial number NCT02644122 for "SF1126 in Recurrent or Progressive SCCHN and Mutations in PIK3CA Gene and/or PI-3 Kinase Pathway Genes" at ClinicalTrials.gov
- Clinical trial number NCT01705847 for "A Phase 1b Study Evaluating GS-9820 in Subjects With Lymphoid Malignancies" at ClinicalTrials.gov
- "Search for clinical trials with GSK2126458 on clinicaltrials.gov". Retrieved 2022-03-17.
- Clinical trial number NCT02260661 for "Phase I, Dose Study to Look at the Safety and Pharmacokinetics of AZD8835 in Patients With Advanced Solid Tumours" at ClinicalTrials.gov
- Clinical trial number NCT01066611 for "Study to Investigate Effects of CAL-263 in Subjects With Allergic Rhinitis Exposed to Allergen in an Environmental Chamber" at ClinicalTrials.gov
- Clinical trial number NCT00695448 for "Phase I Open-Label, Dose-Escalation Study of GSK1059615 in Patients With Solid Tumors or Lymphoma" at ClinicalTrials.gov
- Clinical trial number NCT04495621 for "MEN1611 With Cetuximab in Metastatic Colorectal Cancer (C-PRECISE-01)" at ClinicalTrials.gov
- "Search for phase 1 clinical trials with PWT33597 on clinicaltrials.gov". Retrieved 2022-03-09.
- "Search for phase 1 clinical trials with TG100-115 on clinicaltrials.gov". Retrieved 2022-03-09.
- "Search for phase 1 clinical trials with ZSTK474 on clinicaltrials.gov". Retrieved 2022-03-09.
- Locatelli SL, Stirparo GG, Tartari S, Saba E, Rubino L, Brusamolino E, Castagna L, Santoro A, Carlo-Stella C (2013). "The PI3K/ERK Dual Inhibitor AEZS-136 Induces ROS-Dependent Necroptotic Cell Death and Exerts Potent Antitumor Effects in NOD/SCID Mice with Hodgkin Lymphoma Cell Line Xenografts". Blood. 122 (21): 3067. doi:10.1182/blood.V122.21.3067.3067.
- Zhou H, Yu C, Kong L, Xu X, Yan J, Li Y, et al. (May 2019). "B591, a novel specific pan-PI3K inhibitor, preferentially targets cancer stem cells". Oncogene. 38 (18): 3371–3386. doi:10.1038/s41388-018-0674-5. PMC 6756013. PMID 30635656.
- Heffron TP, Berry M, Castanedo G, Chang C, Chuckowree I, Dotson J, et al. (April 2010). "Identification of GNE-477, a potent and efficacious dual PI3K/mTOR inhibitor". Bioorganic & Medicinal Chemistry Letters. 20 (8): 2408–2411. doi:10.1016/j.bmcl.2010.03.046. PMID 20346656.
- Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, et al. (July 2009). "Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941". Molecular Cancer Therapeutics. 8 (7): 1725–1738. doi:10.1158/1535-7163.MCT-08-1200. PMC 2718129. PMID 19584227.
Further reading
- Williams R, Berndt A, Miller S, Hon WC, Zhang X (August 2009). "Form and flexibility in phosphoinositide 3-kinases". Biochemical Society Transactions. 37 (Pt 4): 615–626. doi:10.1042/BST0370615. PMID 19614567.