Rhenium pentachloride
Rhenium pentachloride is an inorganic compound of chlorine and rhenium. The compound has the formula Re2Cl10 but it is usually referred to as rhenium pentachloride. It is a red-brown solid.
 | |
| Names | |
|---|---|
| IUPAC name
Rhenium pentachloride | |
| Other names
Rhenium(V) chloride, Rhenium chloride, pentachlororhenium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.660 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| ReCl5 | |
| Molar mass | 363.471 g/mol |
| Appearance | red-brown |
| Density | 4.9 g/cm3, solid |
| Melting point | 220 °C (428 °F; 493 K) |
| Boiling point | N/A |
| Will react to decompose and release HCl (g) | |
| +1225.0·10−6 cm3/mol | |
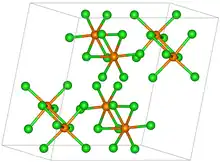
| Structure | |
| Monoclinic, mP48; a = 0.924 nm, b = 1.154 nm, c = 1.203 nm, α = 90°, β = 109.1°, γ = 90° [1] | |
| P21/c, No. 14 | |
| Octahedral | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
releases HCl upon hydrolysis |
| GHS labelling:[2] | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Other anions |
Rhenium hexafluoride |
Related compounds |
Trirhenium nonachloride, rhenium tetrachloride, rhenium hexachloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Structure and preparation
Rhenium pentachloride has a bioctahedral structure and can be formulated as Cl4Re(μ-Cl)2ReCl4. The Re-Re distance is 3.74 Å.[1] The motif is similar to that seen for tantalum pentachloride.
This compound was first prepared in 1933,[3] a few years after the discovery of rhenium. The preparation involves chlorination of rhenium at temperatures up to 900 °C.[4] The material can be purified by sublimation.
ReCl5 is one of the most oxidized binary chlorides of Re. It does not undergo further chlorination. ReCl6 has been prepared from rhenium hexafluoride.[5] Rhenium heptafluoride is known but not the heptachloride.[6]
Uses and reactions
It degrades in air to a brown liquid.[7]
Although rhenium pentachloride has no commercial applications, it is of historic significance as one of the early catalysts for olefin metathesis.[8] Reduction gives trirhenium nonachloride.
Oxygenation affords the Re(VII) oxychloride:[9]
- ReCl5 + 3 Cl2O → ReO3Cl + 5 Cl2
Comproportionation of the penta- and trichloride gives rhenium tetrachloride.
References
- Mucker, K. F.; Smith, G. S.; Johnson, Q. (1968). "The crystal structure of ReCl5" (PDF). Acta Crystallographica Section B. 24 (6): 874. doi:10.1107/S0567740868003316.
- GHS: PubChem 83602
- Geilmann, Wilhelm; Wrigge, Friedrich W.; Biltz, Wilhelm. (1933). "Rheniumpentachlorid". Z. Anorg. Allg. Chem. (in German). 214 (3): 244. doi:10.1002/zaac.19332140304.
- Roger Lincoln, Geoffrey Wilkinson "Rhenium Pentachloride and Volatile Metal Chlorides by Direct Chlorination Using a Vertical-Tube Reactor" Inorganic Syntheses, 1980, Volume 20, Pages 41–43. doi:10.1002/9780470132517.ch11.
- Tamadon, Farhad; Seppelt, Konrad (2013). "The Elusive Halides VCl5, MoCl6, and ReCl6". Angew. Chem. Int. Ed. 52 (2): 767–769. doi:10.1002/anie.201207552. PMID 23172658.
- Stuart A. Macgregor and Klaus H. Moock "Stabilization of High Oxidation States in Transition Metals. 2.1 WCl6 Oxidizes [WF6]-, but Would PtCl6 Oxidize [PtF6]-? An Electrochemical and Computational Study of 5d Transition Metal Halides: [MF6]z versus [MCl6]z (M = Ta to Pt; z = 0, 1−, 2−)" pp 3284–3292. doi:10.1021/ic9605736
- Edwards, D. A.; Ward, R. T. (1970). "Some reactions of rhenium(V) chloride". Journal of the Chemical Society A: 1617. doi:10.1039/J19700001617.
- Ring-opening polymerization of endo and exo-dicyclopentadiene and their 7,8-dihydro derivatives, Hamilton, J.G.; Ivin, K.J.; Rooney, J.J. Journal of Molecular Catalysis 1986 , 36, 115.
- Housecroft, C. E.; Sharpe, A. G. (2004). Inorganic Chemistry (2nd ed.). Prentice Hall. ISBN 978-0-13-039913-7.
