Silver hypochlorite
Silver hypochlorite is an ionic compound of silver and the polyatomic ion hypochlorite.[1] The chemical formula is AgClO.[2] The compound is very unstable and rapidly decomposes.[3]
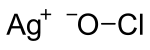 | |
| Names | |
|---|---|
| Other names
Argentous hypochlorite, silver(I) hypochlorite | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| AgClO | |
| Molar mass | 159.32 g·mol−1 |
| very soluble | |
| Related compounds | |
Other anions |
Silver chloride, silver chlorite, silver chlorate, silver perchlorate |
Other cations |
Copper hypochlorite |
Related compounds |
Silver hypobromite, silver hypoiodite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis
- Bubbling chlorine through an aqueous suspension of silver oxide.[4]
- Reaction of hypochlorous acid with silver nitrate produces silver hypochlorite and nitric acid.[5]
Chemical properties
Silver hypochlorite is very unstable, and its solution will soon disproportionate into silver chlorate and silver chloride:
If the AgClO solution is heated to 60 °C, it will rapidly disproportionate. Adding silver oxide stabilizes the solution.[3]
References
- Comey, Arthur Messinger (1896). A Dictionary of Chemical Solubilities; Inorganic. Macmillan and Company. p. 180. Retrieved 10 March 2023.
- "silver hypochlorite". chemsrc.com. Retrieved 10 March 2023.
- Massey, A. G.; Thompson, N. R.; Johnson, B. F. G. (6 June 2016). The Chemistry of Copper, Silver and Gold: Pergamon International Library of Science, Technology, Engineering and Social Studies. Elsevier. p. 108. ISBN 978-1-4831-8169-1. Retrieved 10 March 2023.
- Stas, J. A. (1867). "On the Action of Chlorine on Carbonate of Silver". The Chemical News and Journal of Physical Science: A Journal of Practical Chemistry in All Its Applications to Pharmacy, Arts, and Manufacturers. American Reprint: 173. Retrieved 10 March 2023.
- "Silver Hypochlorite: Formula, Solubility & Molar Mass". study.com. Retrieved 10 March 2023.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.