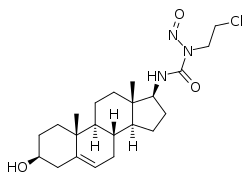
Sturamustine
Sturamustine, also known as dehydroepiandrosterone (DHEA) 17β-N-(2-chloroethyl)-N-nitrosourea, is a synthetic androstane steroid and a C17β nitrosourea conjugate of dehydroepiandrosterone (DHEA) which was developed as a cytostatic antineoplastic agent (i.e., a chemotherapy drug) for the treatment of hormone-dependent tumors but was never marketed.[1][2] It was synthesized in 1982.[1][2]
 | |
| Clinical data | |
|---|---|
| Other names | Dehydroepiandrosterone (DHEA) 17β-N-(2-chloroethyl)-N-nitrosourea; N-(2-Chloroethyl)-N'-(3-hydroxyandrost-5-en-17-yl)-N-nitrosourea |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C22H34ClN3O3 |
| Molar mass | 423.98 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 1122. ISBN 978-1-4757-2085-3.
- Chavis C, de Gourcy C, Borgna JL, Imbach JL (1982). "New steroidal nitrosoureas". Steroids. 39 (2): 129–47. doi:10.1016/0039-128x(82)90081-2. PMID 7071885. S2CID 21042994.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.