Factor D
Factor D (EC 3.4.21.46, C3 proactivator convertase, properdin factor D esterase, factor D (complement), complement factor D, CFD, adipsin) is a protein which in humans is encoded by the CFD gene.[3] Factor D is involved in the alternative complement pathway of the complement system where it cleaves factor B.
| complement factor D (adipsin) | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Identifiers | |||||||
| Symbol | CFD | ||||||
| Alt. symbols | DF, PFD | ||||||
| NCBI gene | 1675 | ||||||
| HGNC | 2771 | ||||||
| OMIM | 134350 | ||||||
| RefSeq | NM_001928 | ||||||
| UniProt | P00746 | ||||||
| Other data | |||||||
| Locus | Chr. 19 p13.3 | ||||||
| |||||||
Function
The protein encoded by this gene is a member of the trypsin family of serine proteases secreted by adipocytes into the bloodstream. The encoded protein is a component of the alternative complement pathway best known for its role in humoral suppression of infectious agents. Finally, the encoded protein has a high level of expression in fat, suggesting a role for adipose tissue in immune system biology.[3]
Factor D is a serine protease that stimulates glucose transport for triglyceride accumulation in fats cells and inhibits lipolysis.[4]
Clinical significance
The level of Factor D is decreased[5] in obese patients. This reduction may be due to high activity or resistance but exact cause is not fully known.
Structure
All members of the chymotrypsin family of serine proteases have very similar structures. In all cases, including factor D, there are two antiparallel β-barrel domains with each barrel containing six β-strands with the same typology in all enzymes. The major difference in backbone structure between Factor D and the other serine proteases of the chymotrypsin family is in the surface loops connecting the secondary structural elements. Factor D displays different conformations of major catalytic and substrate-binding residues typically found in the chrotrypsin family. These features suggest the catalytic activity of factor D is prohibited unless conformational changes are induced by a realignment.[6]
Mechanism of Action
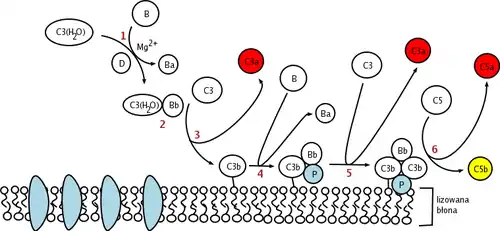
Factor D is a serine protease present in blood and tissue in an active sequence but self-inhibited conformation. The only known natural substrate of Factor D is Factor B, and cleavage of the Arg234-Lys235 scissile bond in Factor B results in two Factor B fragments, Ba and Bb. Before cleavage of the scissile bond in Factor B can occur, Factor B must first bind with C3b before to form the C3bB complex.[7] It is proposed that this conformational change of Factor B in the C3bB complex allows Factor B to fit into the binding site of Factor D.
The catalytic triad of Factor D is composed of Asp102, His57 and Ser195. Other key components of Factor D are an Asp189-Arg218 salt bridge that stabilizes a self-inhibitory loop (amino acid residues 212 to 218) and His57 side chain in the non-canonical conformation.[8][9] In its inhibited form, the self-inhibitory loop prevents access of Factor B to Factor D. When the self-inhibited conformation of Factor D is approached by the C3bB complex, C3bB displaces the salt bridge in Factor D and results in a new salt bridge between the Arg234 of Factor B and Asp189 of Factor D.[10][11] The displacement of the Factor D salt bridge results in a realignment of the self-inhibitory loop and a rotation of the active site histidine side chain, creating the canonical form of Factor D. Cleavage of the scissile bond in Factor B then ensues, releasing fragment Ba and forming C3bBb, the alternative pathway C3-convertase.[12]
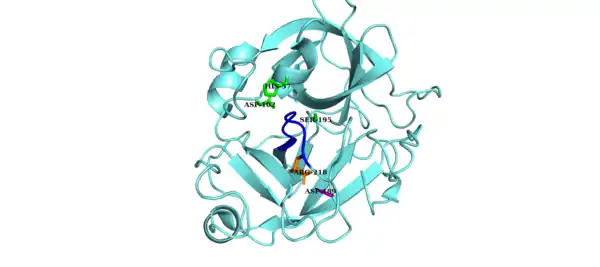 The non-canonical conformation of Factor D is inhibited by the self-inhibitory loop (blue). The Asp-Arg salt bridge (purple and orange side chains, respectively) stabilizes the self-inhibitory loop. The catalytic triad is shown in green.[13] |
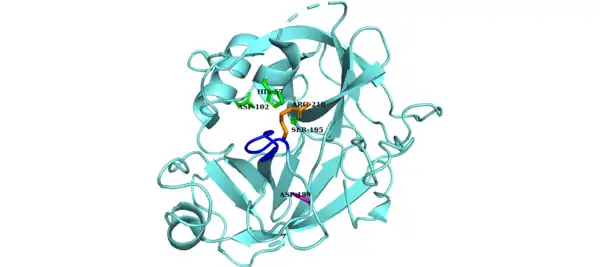 The canonical conformation of Factor D is not self-inhibited. The Asp-Arg salt bridge (purple and orange side chains, respectively) has been displaced resulting in a shift in the self inhibitory loop (blue). The catalytic triad is shown in green.[14] |
Regulation
Factor D is synthesized by the liver and adipocytes with the latter being the major source. The pro-form of Factor D that is secreted is cleaved by MASP-3 to form the active sequence that circulates in the body.[15] Factor D maintains an extremely high substrate specificity, and as a result has no known natural inhibitors in the body.[16] However, most of Factor D remains in the self-inhibited form that limits substrate access to the catalytic site. Factor D has a molecular weight of 23.5 kD and is present at a concentration of 1.8 mg/L of blood in healthy humans. The synthesis rate of Factor is approximately 1.33 mg/kg/day, and most of Factor D is eliminated through the kidney after catabolism in proximal tubules after re-absorption. The net effect is a high fractional metabolic rate of 60% per hour.[17] In patients with normal kidney function, no Factor D was detectable in urine. However, in patients with renal disease, Factor D was found at elevated levels. The alternative pathway is capable of operating even at low levels of Factor D, and deficiencies in levels of Factor D are rare.[18][19]
Role in Diseases
A point mutation resulting in the replacement of a serine codon (Ser42 in the unprocessed methionine form of Factor D) with a stop codon (TAG) in the Factor D gene on chromosome 19 has been documented as a cause of Factor D deficiency.[20] Deficiency in Factor D may cause an increased susceptibility to bacterial infections, specifically Neisseria infections. The mode of inheritance of Factor D deficiency is autosomal recessive, and individuals with a mutation on only one allele may not experience the same susceptibility to reoccurring infections. In a patient with reoccurring infections, complete improvement in the condition was obtained by introducing purified Factor D.[21]
Diseases with excessive complement activation include paroxysmal nocturnal hemoglobinuria (PNH), and inhibitors of Factor D may have utility in the treatment of PNH. Small molecule inhibitors of Factor D are under development for the treatment of PNH, and one small molecule inhibitor, ACH-4471, has shown promise in a Phase 2 clinical trial for Factor D inhibition when combined with eculizumab. Patients treated with Factor D inhibitors must be immunized against infections in order to avoid reoccurring infections as in patients with Factor D deficiency.[22][23]
References
- PDB: 1HFD
- Narayana SV, Carson M, el-Kabbani O, Kilpatrick JM, Moore D, Chen X, Bugg CE, Volanakis JE, DeLucas LJ (1994). "Structure of human factor D. A complement system protein at 2.0 A resolution". Journal of Molecular Biology. 235 (2): 695–708. doi:10.1006/jmbi.1994.1021. PMID 8289289.
- EntrezGene 1675
- Ronti T, Lupattelli G, Mannarino E (2006). "The endocrine function of adipose tissue: an update". Clinical Endocrinology. 64 (4): 355–65. doi:10.1111/j.1365-2265.2006.02474.x. PMID 16584505. S2CID 12455240.
- Flier JS, Cook KS, Usher P, Spiegelman BM (1987). "Severely impaired adipsin expression in genetic and acquired obesity". Science. 237 (4813): 405–8. Bibcode:1987Sci...237..405F. doi:10.1126/science.3299706. PMID 3299706.
- Volanakis JE, Narayana SV (1996). "Complement factor D, a novel serine protease". Protein Science. 5 (4): 553–64. doi:10.1002/pro.5560050401. PMC 2143395. PMID 8845746.
- Lesavre, PH; Müller-Eberhard, HJ (1 December 1978). "Mechanism of action of factor D of the alternative complement pathway". The Journal of Experimental Medicine. 148 (6): 1498–509. doi:10.1084/jem.148.6.1498. PMC 2185104. PMID 82604.
- Jing, H; Babu, YS; Moore, D; Kilpatrick, JM; Liu, XY; Volanakis, JE; Narayana, SV (9 October 1998). "Structures of native and complexed complement factor D: implications of the atypical His57 conformation and self-inhibitory loop in the regulation of specific serine protease activity". Journal of Molecular Biology. 282 (5): 1061–81. doi:10.1006/jmbi.1998.2089. PMID 9753554.
- Jing, H; Macon, KJ; Moore, D; DeLucas, LJ; Volanakis, JE; Narayana, SV (15 February 1999). "Structural basis of profactor D activation: from a highly flexible zymogen to a novel self-inhibited serine protease, complement factor D." The EMBO Journal. 18 (4): 804–14. doi:10.1093/emboj/18.4.804. PMC 1171173. PMID 10022823.
- Karki, RG; Powers, J; Mainolfi, N; Anderson, K; Belanger, DB; Liu, D; Ji, N; Jendza, K; Gelin, CF; Mac Sweeney, A; Solovay, C; Delgado, O; Crowley, M; Liao, SM; Argikar, UA; Flohr, S; La Bonte, LR; Lorthiois, EL; Vulpetti, A; Brown, A; Long, D; Prentiss, M; Gradoux, N; de Erkenez, A; Cumin, F; Adams, C; Jaffee, B; Mogi, M (9 May 2019). "Design, Synthesis, and Preclinical Characterization of Selective Factor D Inhibitors Targeting the Alternative Complement Pathway". Journal of Medicinal Chemistry. 62 (9): 4656–4668. doi:10.1021/acs.jmedchem.9b00271. PMID 30995036. S2CID 122356241.
- Forneris, F; Ricklin, D; Wu, J; Tzekou, A; Wallace, RS; Lambris, JD; Gros, P (24 December 2010). "Structures of C3b in complex with factors B and D give insight into complement convertase formation". Science. 330 (6012): 1816–20. Bibcode:2010Sci...330.1816F. doi:10.1126/science.1195821. PMC 3087196. PMID 21205667.
- Vulpetti, A; Randl, S; Rüdisser, S; Ostermann, N; Erbel, P; Mac Sweeney, A; Zoller, T; Salem, B; Gerhartz, B; Cumin, F; Hommel, U; Dalvit, C; Lorthiois, E; Maibaum, J (9 March 2017). "Structure-Based Library Design and Fragment Screening for the Identification of Reversible Complement Factor D Protease Inhibitors". Journal of Medicinal Chemistry. 60 (5): 1946–1958. doi:10.1021/acs.jmedchem.6b01684. PMID 28157311.
- Maibaum, J; Liao, SM; Vulpetti, A; Ostermann, N; Randl, S; Rüdisser, S; Lorthiois, E; Erbel, P; Kinzel, B; Kolb, FA; Barbieri, S; Wagner, J; Durand, C; Fettis, K; Dussauge, S; Hughes, N; Delgado, O; Hommel, U; Gould, T; Mac Sweeney, A; Gerhartz, B; Cumin, F; Flohr, S; Schubart, A; Jaffee, B; Harrison, R; Risitano, AM; Eder, J; Anderson, K (December 2016). "Small-molecule factor D inhibitors targeting the alternative complement pathway". Nature Chemical Biology. 12 (12): 1105–1110. doi:10.1038/nchembio.2208. PMID 27775713.
- Vulpetti, A; Ostermann, N; Randl, S; Yoon, T; Mac Sweeney, A; Cumin, F; Lorthiois, E; Rüdisser, S; Erbel, P; Maibaum, J (10 May 2018). "Discovery and Design of First Benzylamine-Based Ligands Binding to an Unlocked Conformation of the Complement Factor D." ACS Medicinal Chemistry Letters. 9 (5): 490–495. doi:10.1021/acsmedchemlett.8b00104. PMC 5949727. PMID 29795765.
- Hayashi, M; Machida, T; Ishida, Y; Ogata, Y; Omori, T; Takasumi, M; Endo, Y; Suzuki, T; Sekimata, M; Homma, Y; Ikawa, M; Ohira, H; Fujita, T; Sekine, H (15 September 2019). "Cutting Edge: Role of MASP-3 in the Physiological Activation of Factor D of the Alternative Complement Pathway". Journal of Immunology. 203 (6): 1411–1416. doi:10.4049/jimmunol.1900605. PMID 31399515. S2CID 199518699.
- Lorthiois, E; Anderson, K; Vulpetti, A; Rogel, O; Cumin, F; Ostermann, N; Steinbacher, S; Mac Sweeney, A; Delgado, O; Liao, SM; Randl, S; Rüdisser, S; Dussauge, S; Fettis, K; Kieffer, L; de Erkenez, A; Yang, L; Hartwieg, C; Argikar, UA; La Bonte, LR; Newton, R; Kansara, V; Flohr, S; Hommel, U; Jaffee, B; Maibaum, J (13 July 2017). "Discovery of Highly Potent and Selective Small-Molecule Reversible Factor D Inhibitors Demonstrating Alternative Complement Pathway Inhibition in Vivo". Journal of Medicinal Chemistry. 60 (13): 5717–5735. doi:10.1021/acs.jmedchem.7b00425. PMID 28621538.
- Dobó, J; Kocsis, A; Gál, P (2018). "Be on Target: Strategies of Targeting Alternative and Lectin Pathway Components in Complement-Mediated Diseases". Frontiers in Immunology. 9: 1851. doi:10.3389/fimmu.2018.01851. PMC 6092519. PMID 30135690.
- Volanakis, JE; Barnum, SR; Giddens, M; Galla, JH (14 February 1985). "Renal filtration and catabolism of complement protein D.". The New England Journal of Medicine. 312 (7): 395–9. doi:10.1056/NEJM198502143120702. PMID 3844050.
- Pascual, M; Steiger, G; Estreicher, J; Macon, K; Volanakis, JE; Schifferli, JA (October 1988). "Metabolism of complement factor D in renal failure". Kidney International. 34 (4): 529–36. doi:10.1038/ki.1988.214. PMID 3199673.
- Biesma, DH; Hannema, AJ; van Velzen-Blad, H; Mulder, L; van Zwieten, R; Kluijt, I; Roos, D (July 2001). "A family with complement factor D deficiency". The Journal of Clinical Investigation. 108 (2): 233–40. doi:10.1172/JCI12023. PMC 203023. PMID 11457876.
- Hiemstra, PS; Langeler, E; Compier, B; Keepers, Y; Leijh, PC; van den Barselaar, MT; Overbosch, D; Daha, MR (December 1989). "Complete and partial deficiencies of complement factor D in a Dutch family". The Journal of Clinical Investigation. 84 (6): 1957–61. doi:10.1172/JCI114384. PMC 304077. PMID 2687330.
- Yuan, X; Gavriilaki, E; Thanassi, JA; Yang, G; Baines, AC; Podos, SD; Huang, Y; Huang, M; Brodsky, RA (March 2017). "Small-molecule factor D inhibitors selectively block the alternative pathway of complement in paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome". Haematologica. 102 (3): 466–475. doi:10.3324/haematol.2016.153312. PMC 5394948. PMID 27810992.
- Risitano, AM (January 2014). "Anti-Complement Treatment in Paroxysmal Nocturnal Hemoglobinuria: Where we Stand and Where we are Going". Translational Medicine @ UniSa. 8: 43–52. PMC 4000462. PMID 24778997.
External links
- Complement+Factor+D at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.