Complement component 3
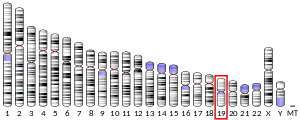
Complement component 3, often simply called C3, is a protein of the immune system. It plays a central role in the complement system and contributes to innate immunity. In humans it is encoded on chromosome 19 by a gene called C3.[5][6]
Function
C3 plays a central role in the activation of the complement system.[7] Its activation is required for both classical and alternative complement activation pathways. People with C3 deficiency are susceptible to bacterial infection.[8][9]
One form of C3-convertase, also known as C4b2a, is formed by a heterodimer of activated forms of C4 and C2. It catalyzes the proteolytic cleavage of C3 into C3a and C3b, generated during activation through the classical pathway as well as the lectin pathway. C3a is an anaphylotoxin and the precursor of some cytokines such as ASP, and C3b serves as an opsonizing agent. Factor I can cleave C3b into C3c and C3d, the latter of which plays a role in enhancing B cell responses. In the alternative complement pathway, C3 is cleaved by C3bBb, another form of C3-convertase composed of activated forms of C3 (C3b) and factor B (Bb). Once C3 is activated to C3b, it exposes a reactive thioester that allows the peptide to covalently attach to any surface that can provide a nucleophile such as a primary amine or a hydroxyl group. Activated C3 can then interact with factor B. Factor B is then activated by factor D, to form Bb. The resultant complex, C3bBb, is called the alternative pathway (AP) C3 convertase.
C3bBb is deactivated in steps. First, the proteolytic component of the convertase, Bb, is removed by complement regulatory proteins having decay-accelerating factor (DAF) activity. Next, C3b is broken down progressively to first iC3b, then C3c + C3dg, and then finally C3d. Factor I is the protease cleaves C3b but requires a cofactor (e.g Factor H, CR1, MCP or C4BP) for activity.
Structure
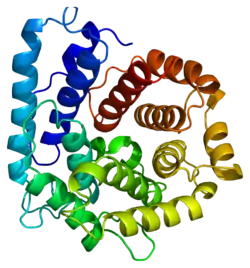
Several crystallographic structures of C3 have been determined[10] and reveal that this protein contains 13 domains.[11][12][13][14]
The C3 precursor protein is first processed by the removal of 4 Arginine residues, forming two chains, beta and alpha, linked by a disulfide bond. The C3 convertase activates C3 by cleaving the alpha chain, releasing C3a anaphylatoxin and generating C3b (beta chain + alpha' (alpha prime) chain).
Biochemistry
Biosynthesis
In humans, C3 is predominantly synthesised by liver hepatocytes[5] and to some degree by epidermis keratinocytes.[15]
Clinical use
| C4 (C) | FB (A) | C3 | CH50 | Conditions |
|---|---|---|---|---|
| · | ↓ | ↓ | ↓ | PSG, C3 NeF AA |
| ↓ | · | ↓ | · | HAE, C4D |
| · | · | · | ↓ | TCPD |
| ↓ | · /↓ | ↓ | ↓ | SLE |
| ↑ | ↑ | ↑ | ↑ | inflammation |
Levels of C3 in the blood may be measured to support or refute a particular medical diagnosis. For example, low C3 levels are associated with Systemic Lupus Erythematosus (SLE)[16] and some types of kidney disease such as post-infectious glomerulonephritis, membranoproliferative glomerulonephritis, and shunt nephritis.
.c3 may be lost in urine in nephrotic syndrome
Pathology
Deficiencies in C3 lead to genetic infections, usually fatal to the newborn.[19]
References
- GRCh38: Ensembl release 89: ENSG00000125730 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000024164 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- de Bruijn MH, Fey GH (Feb 1985). "Human complement component C3: cDNA coding sequence and derived primary structure". Proceedings of the National Academy of Sciences of the United States of America. 82 (3): 708–12. Bibcode:1985PNAS...82..708D. doi:10.1073/pnas.82.3.708. PMC 397115. PMID 2579379.
- "Entrez Gene: C3 complement component 3".
- Sahu A, Lambris JD (Apr 2001). "Structure and biology of complement protein C3, a connecting link between innate and acquired immunity". Immunological Reviews. 180: 35–48. doi:10.1034/j.1600-065X.2001.1800103.x. PMID 11414361. S2CID 21966958.
- Lachmann P (Dec 1975). "Genetics of the complement system". Journal of Medical Genetics. 12 (4): 372–7. doi:10.1136/jmg.12.4.372. PMC 1013316. PMID 768477.
- Matsuyama W, Nakagawa M, Takashima H, Muranaga F, Sano Y, Osame M (Dec 2001). "Molecular analysis of hereditary deficiency of the third component of complement (C3) in two sisters". Internal Medicine. 40 (12): 1254–8. doi:10.2169/internalmedicine.40.1254. PMID 11813855.
- "Advanced search results for UniProt accession P01024". Protein Data Bank in Europe (PDBe). European Bioinformatics Institute. Retrieved 2010-12-27.
- Janssen BJ, Huizinga EG, Raaijmakers HC, Roos A, Daha MR, Nilsson-Ekdahl K, Nilsson B, Gros P (Sep 2005). "Structures of complement component C3 provide insights into the function and evolution of immunity". Nature. 437 (7058): 505–11. Bibcode:2005Natur.437..505J. doi:10.1038/nature04005. hdl:1874/14832. PMID 16177781. S2CID 4344273.
- Janssen BJ, Christodoulidou A, McCarthy A, Lambris JD, Gros P (Nov 2006). "Structure of C3b reveals conformational changes that underlie complement activity". Nature. 444 (7116): 213–6. Bibcode:2006Natur.444..213J. doi:10.1038/nature05172. hdl:1874/20065. PMID 17051160. S2CID 4333755.
- Wiesmann C, Katschke KJ, Yin J, Helmy KY, Steffek M, Fairbrother WJ, McCallum SA, Embuscado L, DeForge L, Hass PE, van Lookeren Campagne M (Nov 2006). "Structure of C3b in complex with CRIg gives insights into regulation of complement activation". Nature. 444 (7116): 217–20. Bibcode:2006Natur.444..217W. doi:10.1038/nature05263. PMID 17051150. S2CID 4372953.
- Fredslund F, Jenner L, Husted LB, Nyborg J, Andersen GR, Sottrup-Jensen L (Aug 2006). "The structure of bovine complement component 3 reveals the basis for thioester function". Journal of Molecular Biology. 361 (1): 115–27. doi:10.1016/j.jmb.2006.06.009. PMID 16831446.
- Pasch, Marcel C.; van den Bosch, Norbert H.A.; Bos, Jan D.; Asghar, Syed S.; Daha, Mohamed R. (January 2000). "Synthesis of Complement Components C3 and Factor B in Human Keratinocytes is Differentially Regulated by Cytokines". Journal of Investigative Dermatology. 114 (1): 78–82. doi:10.1046/j.1523-1747.2000.00841.x. PMID 10620119. Retrieved 28 August 2017.
- "Complement C3 (Blood) - Health Encyclopedia - University of Rochester Medical Center".
- Soames CJ, Sim RB (Sep 1997). "Interactions between human complement components factor H, factor I and C3b". The Biochemical Journal. 326 (2): 553–61. doi:10.1042/bj3260553. PMC 1218704. PMID 9291131.
- Jokiranta TS, Westin J, Nilsson UR, Nilsson B, Hellwage J, Löfås S, Gordon DL, Ekdahl KN, Meri S (Mar 2001). "Complement C3b interactions studied with surface plasmon resonance technique". International Immunopharmacology. 1 (3): 495–506. doi:10.1016/S1567-5769(00)00042-4. PMID 11367533.
- Abbas, Abul K.; Lichtman, Andrew H.; Pillai, Shiv (2012). "Chapter 8: Effector Mechanisms of Humoral Immunity". Basic Immunology: Functions and Disorders of the Immune System (Fourth ed.). Philadelphia, PA: Elsevier Saunders. p. 162. ISBN 978-1-4557-0707-2.
Further reading
- Bradley DT, Zipfel PF, Hughes AE (Jun 2011). "Complement in age-related macular degeneration: a focus on function". Eye. 25 (6): 683–93. doi:10.1038/eye.2011.37. PMC 3178140. PMID 21394116.
External links
- GeneReviews/NCBI/NIH/UW entry on Atypical Hemolytic-Uremic Syndrome
- OMIM entries on Atypical Hemolytic-Uremic Syndrome
- GeneReviews/NCBI/NIH/UW entry on Dense Deposit Disease/Membranoproliferative Glomerulonephritis Type II
- Complement+C3 at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P01024 (Complement C3) at the PDBe-KB.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.