Bacillus cereus
Bacillus cereus is a Gram-positive, rod-shaped, facultatively anaerobic, motile, beta-hemolytic, spore-forming bacterium commonly found in soil, food and marine sponges.[1] The specific name, cereus, meaning "waxy" in Latin, refers to the appearance of colonies grown on blood agar. Some strains are harmful to humans and cause foodborne illness, while other strains can be beneficial as probiotics for animals.[2][3] B. cereus bacteria are facultative anaerobes, and like other members of the genus Bacillus, can produce protective endospores. Its virulence factors include phospholipase C, cereulide, sphingomyelinase, metalloproteases, and cytotoxin K.[4]
| Bacillus cereus | |
|---|---|
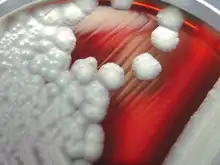 | |
| B. cereus colonies on a sheep-blood agar plate | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Bacilli |
| Order: | Bacillales |
| Family: | Bacillaceae |
| Genus: | Bacillus |
| Species: | B. cereus |
| Binomial name | |
| Bacillus cereus Frankland & Frankland 1887 | |
| Biovars | |

The Bacillus cereus group comprises seven closely related species: B. cereus sensu stricto (referred to herein as B. cereus), B. anthracis, B. thuringiensis, B. mycoides, B. pseudomycoides, and B. cytotoxicus;[5] or as six species in a Bacillus cereus sensu lato: B. weihenstephanensis, B. mycoides, B. pseudomycoides, B. cereus, B. thuringiensis, and B. anthracis.[6]
A phylogenomic analysis combined with average nucleotide identity (ANI) analysis revealed that the B. anthracis species also includes strains annotated as B. cereus and B. thuringiensis.[7]
The three most important species within the B. cereus group include B. anthracis, B. thuringiensis, and B. cereus. These demonstrate a wide variety of different phenotypes and pathological effects.
Diseases related to B. cereus are growing in number as they are found in particular food poisoning.
History
Colonies of B. cereus were originally isolated from an agar plate left exposed to the air in a cow shed.[8] In the 2010s, examination of warning letters issued by the US Food and Drug Administration issued to pharmaceutical manufacturing facilities addressing facility microbial contamination revealed that the most common contaminant was B. cereus.[9]
Several new enzymes have been discovered in B. cereus, such as AlkC and AlkD, both of which are involved in DNA repair.[10]
Ecology
Like most Bacilli, the most common ecosystem of Bacillus cereus is the soil. In concert with Arbuscular mycorrhiza (and Rhizobium leguminosarum in clover), they can up-regulate plant growth in heavy metal soils by decreasing heavy metal concentrations via bioaccumulation and biotransformation in addition to increasing phosphorus, nitrogen, and potassium uptake in certain plants.[11] B. cereus was also shown to aid in survival of earthworms in heavy metal soils resulting from the use of metal-based fungicides, showing increases in biomass, reproduction and reproductive viability, and a decrease in metal content of tissues in those inoculated with the bacterium.[12] These results suggest strong possibilities for its application in ecological bioremediation.
B. cereus competes with Gram-negative bacteria species such as Salmonella and Campylobacter in the gut; its presence reduces the number of Gram-negative bacteria, specifically via antibiotic activity via enzymes that impede their quorum sensing ability and exhibit bactericidal activity.[13] In food animals such as chickens,[14] rabbits[15] and pigs,[16] some harmless strains of B. cereus are used as a probiotic feed additive to reduce Salmonella in the animals' intestines and cecum. This improves the animals' growth, as well as food safety for humans who eat them. B. cereus can parasitize codling moth larvae. In addition, B. cereus create and release enzymes that aid in the digestion of materials that are typically difficult to digest, such as woody plant matter, in the guts of other organisms.[13]
B. cereus and other members of Bacillus are not easily killed by alcohol; they have been known to colonize distilled liquors and alcohol-soaked swabs and pads in numbers sufficient to cause infection.[17][18]
Some strains of B. cereus produce cereins, bacteriocins active against different B. cereus strains or other Gram-positive bacteria.[19]
B. cereus and other Bacillus species are ubiquitous organisms; present in all environments.
In agriculture
B. cereus B25 is a biofungicide.[20] Figueroa-López et al. 2016 reduce Fusarium verticillioides growth using this strain.[20] B25 shows promise for reduction of mycotoxin concentrations in grains.[20]
Reproduction
At 30 °C (86 °F), a population of B. cereus can double in as little as 20 minutes or as long as 3 hours, depending on the food product.[21]
| Food | Minutes to double, 30 °C (86 °F) | Hours to multiply by 1,000,000 |
|---|---|---|
| Milk | 20–36 | 6.6 - 12 |
| Cooked rice | 26–31 | 8.6 - 10.3 |
| Infant formula | 56 | 18.6 |
Pathogenesis
B. cereus is responsible for a minority of foodborne illnesses (2–5%), causing severe nausea, vomiting, and diarrhea.[22] Bacillus foodborne illnesses occur due to survival of the bacterial endospores when infected food is not, or is inadequately, cooked.[23] Cooking temperatures less than or equal to 100 °C (212 °F) allow some B. cereus spores to survive.[24] This problem is compounded when food is then improperly refrigerated, allowing the endospores to germinate.[25] Cooked foods not meant for either immediate consumption or rapid cooling and refrigeration should be kept at temperatures below 10 °C (50 °F) or above 50 °C (122 °F).[24] Germination and growth generally occur between 10 °C and 50 °C,[24] though some strains can grow at low temperatures,[26] and Bacillus cytotoxicus strains have been shown to grow at temperatures up to 52℃.[27] Bacterial growth results in production of enterotoxins, one of which is highly resistant to heat and acids (pH levels between 2 and 11);[28] ingestion leads to two types of illness: diarrheal and emetic (vomiting) syndrome.[29]
- The diarrheal type is associated with a wide range of foods, has an 8-to-16-hour incubation time, and is associated with diarrhea and gastrointestinal pain. Also known as the 'long-incubation' form of B. cereus food poisoning, it might be difficult to differentiate from poisoning caused by Clostridium perfringens.[28] Enterotoxin can be inactivated after heating at 56 °C (133 °F) for 5 minutes, but whether its presence in food causes the symptom is unclear, since it degrades in stomach enzymes; its subsequent production by surviving B. cereus spores within the small intestine may be the cause of illness.[30]
- The 'emetic' form commonly results from rice which is cooked at a time and temperature insufficient to kill any spores present, then improperly refrigerated. The remaining spores can produce a toxin, cereulide, which is not inactivated by later reheating. This form leads to nausea and vomiting 1–5 hours after consumption. Distinguishing from other short-term bacterial foodborne intoxications, such as by Staphylococcus aureus, can be difficult.[31] Emetic toxin can withstand 121 °C (250 °F) for 90 minutes.[32]
The diarrhetic syndromes observed in patients are thought to stem from the three toxins: hemolysin BL (Hbl), nonhemolytic enterotoxin (Nhe), and cytotoxin K (CytK).[33] The nhe/hbl/cytK genes are located on the chromosome of the bacteria. Transcription of these genes is controlled by PlcR. These genes occur in the taxonomically related B. thuringiensis and B. anthracis, as well. These enterotoxins are all produced in the small intestine of the host, thus thwarting digestion by host endogenous enzymes. The Hbl and Nhe toxins are pore-forming toxins closely related to ClyA of E. coli. The proteins exhibit a conformation known as a "beta-barrel" that can insert into cellular membranes due to a hydrophobic exterior, thus creating pores with hydrophilic interiors. The effect is loss of cellular membrane potential and eventually cell death. CytK is a pore-forming protein more related to other hemolysins.
Previously, it was thought that the timing of the toxin production was responsible for the two different courses of disease, but it has since been found that the emetic syndrome is caused by the toxin cereulide, which is found only in emetic strains and is not part of the "standard toolbox" of B. cereus. Cereulide is a cyclic polypeptide containing three repeats of four amino acids: D-oxy-Leu—D-Ala—L-oxy-Val—L-Val (similar to valinomycin produced by Streptomyces griseus) produced by nonribosomal peptide synthesis. Cereulide is believed to bind to 5-hydroxytryptamine 3 (5-HT3) serotonin receptors, activating them and leading to increased afferent vagus nerve stimulation.[34] It was shown independently by two research groups to be encoded on multiple plasmids: pCERE01[35] or pBCE4810.[36] Plasmid pBCE4810 shares homology with the B. anthracis virulence plasmid pXO1, which encodes the anthrax toxin. Periodontal isolates of B. cereus also possess distinct pXO1-like plasmids. Like most of cyclic peptides containing nonproteogenic amino acids, cereulide is resistant to heat, proteolysis, and acid conditions.[37]
B. cereus is also known to cause difficult-to-eradicate chronic skin infections, though less aggressive than necrotizing fasciitis. B. cereus can also cause keratitis.[38]
While often associated with gastrointestinal illness, B. cereus is also associated with illnesses such as fulminant bacterial infection, central nervous system involvement, respiratory tract infection, and endophthalmitis. While different from B. anthracis, B. cereus contains some toxin genes originally found in B. anthracis that are attributed to anthrax-like respiratory tract infections. [39]
A case study was published in 2019 of a catheter-related bloodstream infection of B. cereus in a 91-year-old male previously being treated with hemodialysis via PermCath for end-stage renal disease.[40] He presented with chills, tachypnea, and high-grade fever, his white blood cell count and high-sensitivity C-reactive protein (CRP) were significantly elevated, and CT imaging revealed a thoracic aortic aneurysm. He was successfully treated for the aneurysm with intravenous vancomycin, oral fluoroquinolones, and PermCath removal. The pathogenicity of B. cereus in gastrointestinal and nongastrointestinal infections is associated with the ability to produce toxins.
Some virulence factors of B. cereus include toxins, beta-lactamase, collagenase, and other enzymes.
Spore elimination
While B. cereus vegetative cells are killed during normal cooking, spores are more resistant. Viable spores in food can become vegetative cells in the intestines and produce a range of diarrheal enterotoxins, so elimination of spores is desirable. In wet heat (poaching, simmering, boiling, braising, stewing, pot roasting, steaming), spores require more than 5 minutes at 121 °C (250 °F) at the coldest spot to be destroyed. In dry heat (grilling, broiling, baking, roasting, searing, sautéing), 120 °C (248 °F) for 1 hour kills all spores on the exposed surface.[41]
Diagnosis
In case of foodborne illness, the diagnosis of B. cereus can be confirmed by the isolation of more than 100,000 B. cereus organisms per gram from epidemiologically-implicated food, but such testing is often not done because the illness is relatively harmless and usually self-limiting.[42]
Identification
For the isolation and enumeration of B. cereus, there are two standardized methods by International Organization for Standardization (ISO): ISO 7932 and ISO 21871. Because of B. cereus' ability to produce lecithinase and its inability to ferment mannitol, there are some proper selective media for its isolation and identification such as mannitol-egg yolk-polymyxin (MYP) and polymyxin-pyruvate-egg yolk-mannitol-bromothymol blue agar (PEMBA). B. cereus colonies on MYP have a violet-red background and are surrounded by a zone of egg-yolk precipitate.[43]
Below is a list of differential techniques and results that can help to identify B. cereus from other bacteria and Bacillus species.[44]
- Anaerobic growth: Positive
- Voges Proskauer test: Positive
- Acid produced from
- D-glucose: Positive
- L-arabinose: Negative
- D-xylose: Negative
- D-mannitol: Negative
- Starch hydrolysis: Positive
- Nitrate reduction: Positive
- Degradation of tyrosine: Positive
- Growth at
- above 50 °C: Negative
- Use of citrate: Positive
The Central Public Health Laboratory in the United Kingdom tests for motility, hemolysis, rhizoid growth, susceptibility to γ-phage, and fermentation of ammonium salt-based glucose but no mannitol, arabinose, or xylose.[43]
Prognosis
Most emetic patients recover within 6 to 24 hours,[29] but in some cases, the toxin can be fatal via fulminant hepatic failure.[45][46][47][48][49] In 2014, 23 newborns in the UK receiving total parenteral nutrition contaminated with B. cereus developed septicaemia, with three of the infants later dying as a result of infection.[50][51]
Diseases in Aquatic animals
Bacillus cereus groups, notably B. cereus (Bc) and B. thuringiensis (Bt)[52], are also pathogenic to multiple aquatic organisms including Chinese softshell turtle ( Pelodiscus sinensis ), causing infection characterized by gross lesions such as hepatic congestion and enlarged spleen, which causes high mortality.
Characteristics of B. cereus
Colony, morphological, physiological, and biochemical characteristics of marine B. cereus are shown in the Table below.[1]
| Test type | Test | Characteristics |
| Colony characters | Size | Medium |
| Type | Round | |
| Color | Whitish | |
| Shape | Convex | |
| Morphological characters | Shape | Rod |
| Physiological characters | Motility | + |
| Growth at 6.5% NaCl | + | |
| Biochemical characters | Gram's staining | + |
| Oxidase | + | |
| Catalase | + | |
| Oxidative-Fermentative | Fermentative | |
| Motility | + | |
| Methyl Red | – | |
| Voges-Proskauer | + | |
| Indole | – | |
| H2S Production | – | |
| Urease | V | |
| Nitrate reductase | + | |
| β-Galactosidase | – | |
| Hydrolysis of | Gelatin | + |
| Aesculin | + | |
| Casein | + | |
| Tween 40 | + | |
| Tween 60 | + | |
| Tween 80 | + | |
| Acid production from | Glycerol | + |
| Galactose | V | |
| D-Glucose | + | |
| D-Fructose | + | |
| D-Mannose | – | |
| Mannitol | + | |
| N-Acetylglucosamine | + | |
| Amygdalin | + | |
| Maltose | + | |
| D-Melibiose | + | |
| D-Trehalose | + | |
| Glycogen | + | |
| D-Turanose | V |
Note: + = Positive, – =Negative, V= Variable (+/–)
Pathogens of B. Cereus
Bacteria of the B. cereus group are infected by bacteriophages belonging to the family Tectiviridae. This family includes tailless phages that have a lipid membrane or vesicle beneath the icosahedral protein shell and that are formed of approximately equal amounts of virus-encoded proteins and lipids derived from the host cell's plasma membrane. Upon infection, the lipid membrane becomes a tail-like structure used in genome delivery. The genome is composed of about 15-kilobase, linear, double-stranded DNA (dsDNA) with long, inverted terminal-repeat sequences (100 base pairs). GIL01, Bam35, GIL16, AP50, and Wip1 are examples of temperate tectiviruses infecting the B. cereus group.[53]
See also
References
- Paul, Sulav Indra; Rahman, Md. Mahbubur; Salam, Mohammad Abdus; Khan, Md. Arifur Rahman; Islam, Md. Tofazzal (15 December 2021). "Identification of marine sponge-associated bacteria of the Saint Martin's island of the Bay of Bengal emphasizing on the prevention of motile Aeromonas septicemia in Labeo rohita". Aquaculture. 545: 737156. doi:10.1016/j.aquaculture.2021.737156. ISSN 0044-8486.
- Ryan, Kenneth J.; Ray, C. George, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- Felis, Giovanna E.; Dellaglio, Franco; Torriani, Sandra (2009). "Taxonomy of probiotic microorganisms". In Charalampopoulos, Dimitris; Rastall, Robert A. (eds.). Prebiotics and Probiotics Science and Technology. Springer Science & Business Media. p. 627. ISBN 978-0-387-79057-2.
- Tuipulotu, Daniel Enosi; Mathur, Anukriti; Ngo, Chinh; Man, Si Ming (1 May 2021). "Bacillus cereus: Epidemiology, Virulence Factors, and Host–Pathogen Interactions". Trends in Microbiology. 29 (5): 458–471. doi:10.1016/j.tim.2020.09.003. ISSN 0966-842X. PMID 33004259.
- Guinebretière, Marie-Hélène; Auger, Sandrine; Galleron, Nathalie; Contzen, Matthias; et al. (2013). "Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus Group occasionally associated with food poisoning". International Journal of Systematic and Evolutionary Microbiology. 63 (1): 31–40. doi:10.1099/ijs.0.030627-0. PMID 22328607. S2CID 2407509.
- Kolstø, Anne-Brit; Tourasse, Nicolas J.; Økstad, Ole Andreas (2009). "What Sets Bacillus anthracis Apart from Other Bacillus Species?". Annual Review of Microbiology. Annual Reviews. 63 (1): 451–476. doi:10.1146/annurev.micro.091208.073255. ISSN 0066-4227. PMID 19514852.
- Nikolaidis, Marios; Hesketh, Andrew; Mossialos, Dimitris; Iliopoulos, Ioannis; Oliver, Stephen G.; Amoutzias, Grigorios D. (26 August 2022). "A Comparative Analysis of the Core Proteomes within and among the Bacillus subtilis and Bacillus cereus Evolutionary Groups Reveals the Patterns of Lineage- and Species-Specific Adaptations". Microorganisms. 10 (9): 1720. doi:10.3390/microorganisms10091720. ISSN 2076-2607. PMC 9505155. PMID 36144322.
- Frankland, Grace C.; Frankland, Percy Faraday (1 January 1887). "Studies on some new micro-organisms obtained from air". Philosophical Transactions of the Royal Society B: Biological Sciences. 178: 257–287. Bibcode:1887RSPTB.178..257F. doi:10.1098/rstb.1887.0011. JSTOR 91702.
- Sandle, Tim (28 November 2014). "The risk of Bacillus cereus to pharmaceutical manufacturing". American Pharmaceutical Review (Paper). 17 (6): 56. Archived from the original on 25 April 2015.
- Alseth, Ingrun; Rognes, Torbjørn; Lindbäck, Toril; Solberg, Inger; et al. (2006). "A new protein superfamily includes two novel 3-methyladenine DNA glycosylases Bacillus cereus, AlkC and AlkD". Molecular Microbiology. 59 (5): 1602–1609. doi:10.1111/j.1365-2958.2006.05044.x. PMC 1413580. PMID 16468998.
- Azcón, Rosario; Perálvarez, María de Carmen; Roldán, Antonio; Barea, José-Miguel (16 December 2009). "Arbuscular Mycorrhizal Fungi, Bacillus cereus, and Candida parapsilosis from a Multicontaminated Soil Alleviate Metal Toxicity in Plants". Microbial Ecology. 59: 668–677. doi:10.1007/s00248-009-9618-5.
- Oladipo, Oluwatosin G.; Burt, Adam F.; Maboeta, Mark S. (1 January 2019). "Effect of Bacillus cereus on the ecotoxicity of metal-based fungicide spiked soils: Earthworm bioassay". Ecotoxicology. 28 (1): 37–47. doi:10.1007/s10646-018-1997-2. ISSN 1573-3017.
- Swiecicka, Izabela (1 January 2008). "Natural occurrence of Bacillus thuringiensis and Bacillus cereus in eukaryotic organisms: a case for symbiosis". Biocontrol Science and Technology. 18 (3): 221–239. doi:10.1080/09583150801942334. ISSN 0958-3157.
- Vilà, B.; Fontgibell, A.; Badiola, I.; Esteve-Garcia, E.; et al. (2009). "Reduction of Salmonella enterica var. enteritidis colonization and invasion by Bacillus cereus var. toyoi inclusion in poultry feeds". Poultry Science. 88 (55): 975–979. doi:10.3382/ps.2008-00483. PMID 19359685.
- Bories, Georges; Brantom, Paul; de Barberà, Joaquim Brufau; Chesson, Andrew; et al. (9 December 2008). "Safety and efficacy of the product Toyocerin (Bacillus cereus var. toyoi) as feed additive for rabbit breeding does". EFSA Journal. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed. 2009 (1): 913. doi:10.2903/j.efsa.2009.913. eISSN 1831-4732. EFSA-Q-2008-287. Retrieved 14 May 2009.
- Bories, Georges; Brantom, Paul; de Barberà, Joaquim Brufau; Chesson, Andrew; et al. (16 March 2007). "Opinion of the Scientific Panel on Additives and Products or Substances used in Animal Feed on the safety and efficacy of the product Toyocerin (Bacillus cereus var. Toyoi) as a feed additive for sows from service to weaning, in accordance with Regulation (EC) No 1831/2003". EFSA Journal. Scientific Opinion of the Panel on Additives and Products or Substances used in Animal Feed. 2007 (3): 458. doi:10.2903/j.efsa.2007.458. eISSN 1831-4732. EFSA-Q-2006-037. Retrieved 14 May 2009.
- "Notes from the Field: Contamination of alcohol prep pads with Bacillus cereus group and Bacillus species — Colorado, 2010". CDC. 25 March 2011. Archived from the original on 1 July 2018.
- Hsueh, Po-Ren; Teng, Lee-Jene; Yang, Pan-Chyr; Pan, Hui-Lu; et al. (1999). "Nosocomial pseudoepidemic caused by Bacillus cereus traced to contaminated ethyl alcohol from a liquor factory". Journal of Clinical Microbiology. 37 (7): 2280–2284. doi:10.1128/JCM.37.7.2280-2284.1999. PMC 85137. PMID 10364598.
- Naclerio, Gino; Ricca, Ezio; Sacco, Margherita; De Felice, Maurilio (December 1993). "Antimicrobial activity of a newly identified bacteriocin of Bacillus cereus". Applied and Environmental Microbiology. 59 (12): 4313–4316. Bibcode:1993ApEnM..59.4313N. doi:10.1128/AEM.59.12.4313-4316.1993. PMC 195902. PMID 8285719.
-
- Verma, Maya; Mishra, Jitendra; Arora, Naveen Kumar (2019). "Plant Growth-Promoting Rhizobacteria: Diversity and Applications". In Sobti, Ranbir Chander; Arora, Naveen Kumar; Kothari, Richa (eds.). Environmental Biotechnology: For Sustainable Future. Springer Singapore. doi:10.1007/978-981-10-7284-0_6. S2CID 91258998.
- Ndemera, Melody; De Boevre, Marthe; De Saeger, Sarah (2020). "Mycotoxin management in a developing country context: A critical review of strategies aimed at decreasing dietary exposure to mycotoxins in Zimbabwe". Critical Reviews in Food Science and Nutrition. Taylor & Francis. 60 (4): 529–540. doi:10.1080/10408398.2018.1543252. ISSN 1040-8398. PMID 30501517. S2CID 54523328.
- Verma, Rishi Kumar; Sachan, Manisha; Vishwakarma, Kanchan; Upadhyay, Neha; Mishra, Rohit Kumar; Tripathi, Durgesh Kumar; Sharma, Shivesh (2018). "Role of PGPR in Sustainable Agriculture: Molecular Approach Toward Disease Suppression and Growth Promotion". Role of Rhizospheric Microbes in Soil. Singapore: Springer Singapore. pp. 259–290. doi:10.1007/978-981-13-0044-8_9. ISBN 978-981-13-0043-1. S2CID 90538241.
- Shahid, Mohammad; Zaidi, Almas; Khan, Mohd. Saghir; Rizvi, Asfa; Saif, Saima; Ahmed, Bilal (2017). "Recent Advances in Management Strategies of Vegetable Diseases". Microbial Strategies for Vegetable Production. Cham, Switzerland: Springer International Publishing. pp. 197–226. doi:10.1007/978-3-319-54401-4_9. ISBN 978-3-319-54400-7. S2CID 91152604.
- Figueroa-López, Alejandro Miguel; Cordero-Ramírez, Jesús Damián; Martínez-Álvarez, Juan Carlos; López-Meyer, Melina; Lizárraga-Sánchez, Glenda Judith; Félix-Gastélum, Rubén; Castro-Martínez, Claudia; Maldonado-Mendoza, Ignacio Eduardo (2016). "Rhizospheric bacteria of maize with potential for biocontrol of Fusarium verticillioides". SpringerPlus. 5 (330). doi:10.1186/s40064-016-1780-x. S2CID 12268357.
- Mikkola, Raimo (2006). Food and indoor air isolated Bacillus non-protein toxins: structures, physico-chemical properties and mechanisms of effects on eukaryotic cells (PDF) (Thesis). University of Helsinki. p. 12. ISBN 952-10-3549-8. Archived (PDF) from the original on 9 July 2019. Retrieved 24 October 2015.
- Kotiranta, Anja; Lounatmaa, Kari; Haapasalo, Markus (February 2000). "Epidemiology and pathogenesis of Bacillus cereus infections". Microbes and Infection. 2 (2): 189–198. doi:10.1016/S1286-4579(00)00269-0. PMID 10742691.
- Turnbull, Peter C. B. (1996). "Bacillus". In Baron S.; et al. (eds.). Baron's Medical Microbiology (4th ed.). University of Texas Medical Branch. ISBN 978-0-9631172-1-2 – via NCBI Bookshelf.
- Roberts, T. A.; Baird-Parker, A. C.; Tompkin, R. B. (1996). Characteristics of Microbial Pathogens. London: Blackie Academic & Professional. p. 24. ISBN 978-0-412-47350-0. Retrieved 25 November 2010.
- McKillip, John L. (May 2000). "Prevalence and expression of enterotoxins in Bacillus cereus and other Bacillus spp., a literature review". Antonie van Leeuwenhoek. 77 (4): 393–399. doi:10.1023/A:1002706906154. PMID 10959569. S2CID 8362130.
- Lawley, Richard; Curtis, Laurie; Davis, Judy (2008). The Food Safety Hazard Guidebook. Cambridge, UK: Royal Society of Chemistry. p. 17. ISBN 978-0-85404-460-3. Retrieved 25 November 2010.
- Cairo, Jessica; Gherman, Iulia; Day, Andrew; Cook, Paul E. (2022). "Bacillus cytotoxicus —A potentially virulent food‐associated microbe". Journal of Applied Microbiology. 132 (1): 31–40. doi:10.1111/jam.15214. PMID 34260791. S2CID 235906633.
- Todar, Kenneth. "Bacillus cereus". Todar's Online Textbook of Bacteriology. Retrieved 19 September 2009.
- Ehling-Shulz, Monika; Fricker, Martina; Scherer, Siegfried (19 November 2004). "Bacillus cereus, the causative agent of an emetic type of food-borne illness". Molecular Nutrition & Food Research. 48 (7): 479–487. doi:10.1002/mnfr.200400055. PMID 15538709.
- Millar, Ian; Gray, David; Kay, Helen (1998). "Bacterial toxins found in foods". In Watson, David H. (ed.). Natural Toxicants in Food. CRC Press. pp. 133–134. ISBN 978-0-8493-9734-9.
- Todar, Kenneth. "Bacillus cereus". Todar's Online Textbook of Bacteriology. Retrieved 19 September 2009.
- Millar, Ian; Gray, David; Kay, Helen (1998). "Bacterial toxins found in foods". In Watson, David H. (ed.). Natural Toxicants in Food. CRC Press. pp. 133–134. ISBN 978-0-8493-9734-9.
- Guinebretière, Marie-Hélène; Broussolle, Véronique; Nguyen-The, Christophe (August 2002). "Enterotoxigenic profiles of food-poisoning and food-borne Bacillus cereus strains". Journal of Clinical Microbiology. 40 (8): 3053–3056. doi:10.1128/JCM.40.8.3053-3056.2002. PMC 120679. PMID 12149378.
- Agata, Norio; Ohta, Michio; Mori, Masashi; Isobe, Minoru (June 1995). "A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus". FEMS Microbiology Letters. 129 (1): 17–20. doi:10.1016/0378-1097(95)00119-P. PMID 7781985.
- Hoton, Florence M.; Andrup, Lars; Swiecicka, Izabela; Mahillon, Jacques (July 2005). "The cereulide genetic determinants of emetic Bacillus cereus are plasmid-borne". Microbiology. 151 (7): 2121–2124. doi:10.1099/mic.0.28069-0. PMID 16000702.
- Ehling-Shulz, Monika; Fricker, Martina; Grallert, Harald; Rieck, Petra; et al. (2 March 2006). "Cereulide synthetase gene cluster from emetic Bacillus cereus: Structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1". BMC Microbiology. 6: 20. doi:10.1186/1471-2180-6-20. PMC 1459170. PMID 16512902.
- Stenfors Arnesen, Lotte P.; Fagerlund, Annette; Granum, Per Einar (1 July 2008). "From soil to gut: Bacillus cereus and its food poisoning toxins". FEMS Microbiology Reviews. 32 (4): 579–606. doi:10.1111/j.1574-6976.2008.00112.x. PMID 18422617.
- Pinna, Antonio; Sechi, Leonardo A.; Zanetti, Stefania; Usai, Donatella; et al. (October 2001). "Bacillus cereus keratitis associated with contact lens wear". Ophthalmology. 108 (10): 1830–1834. doi:10.1016/S0161-6420(01)00723-0. PMID 11581057.
- Bottone, E. J. (1 April 2010). "Bacillus cereus, a Volatile Human Pathogen". Clinical Microbiology Reviews. 23 (2): 382–398 – via ASM Journals.
- Wu, Tzu-Chi; Pai, Ching-Chou; Huang, Pin-Wen; Tung, Chun-Bin (11 November 2019). "Infected aneurysm of the thoracic aorta probably caused by Bacillus cereus: a case report". BMC Infectious Diseases. 19 (1): 959. doi:10.1186/s12879-019-4602-2. ISSN 1471-2334. PMC 6849281. PMID 31711418.
- Bremer, Phil (3 October 2016). "Bacillus spores in the food industry: A review on resistance and response to novel inactivation technologies". Comprehensive Reviews in Food Science and Food Safety. 15 (2016): 1139–1148. doi:10.1111/1541-4337.12231. PMID 33401831.
- "Bacillus cereus food poisoning associated with fried rice at two child day care centers" (PDF). Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention. 43 (10). 18 March 1994. Archived (PDF) from the original on 9 October 2022.
- Griffiths, M.W.; Schraft, H. (2017). "Bacillus cereus food poisoning". In Dodd, Christine E. R.; et al. (eds.). Foodborne Diseases (3rd ed.). Elsevier. pp. 395–405. doi:10.1016/b978-0-12-385007-2.00020-6. ISBN 978-0-12-385007-2.
- Harwood, Colin R., ed. (1989). Bacillus. Springer Science. ISBN 978-1-4899-3502-1. OCLC 913804139.
- Takabe, Fukutaro; Oya, Masakazu (March–April 1976). "An autopsy case of food poisoning associated with Bacillus cereus". Forensic Science. 7 (2): 97–101. doi:10.1016/0300-9432(76)90024-8. PMID 823082.
- Mahler, Hellmut; Pasi, Aurelio; Kramer, John M.; Schulte, Petra; et al. (17 April 1997). "Fulminant liver failure in association with the emetic toxin of Bacillus cereus". The New England Journal of Medicine. 336 (16): 1142–1148. doi:10.1056/NEJM199704173361604. PMID 9099658.
- Dierick, Katelijne; Van Coillie, Els; Swiecicka, Izabela; Meyfroidt, Geert; et al. (August 2005). "Fatal family outbreak of Bacillus cereus-associated food poisoning". Journal of Clinical Microbiology. 43 (8): 4277–4279. doi:10.1128/JCM.43.8.4277-4279.2005. PMC 1233987. PMID 16082000.
- Shiota, Mitsutaka; Saitou, Keiko; Mizumoto, Hiroshi; Matsusaka, Masanori; et al. (April 2010). "Rapid detoxification of cereulide in Bacillus cereus food poisoning". Pediatrics. 125 (4): e951–e955. doi:10.1542/peds.2009-2319. PMID 20194285. S2CID 19744459.
- Naranjo, María; Denayer, Sarah; Botteldoorn, Nadine; Delbrassinne, Laurence; et al. (December 2011). "Sudden death of a young adult associated with Bacillus cereus food poisoning". Journal of Clinical Microbiology. 49 (12): 4379–4381. doi:10.1128/JCM.05129-11. PMC 3232990. PMID 22012017.
- "Medical safety alert: Lipid Phase only of Parenteral Nutrition – potential contamination with Bacillus cereus". UK Medicines and Healthcare products Regulatory Agency. 4 June 2014.
- Cooper, Charlie (1 July 2014). "Third baby dies from contaminated 'Total Parenteral Nutrition' drip feed". The Independent. Archived from the original on 18 April 2019.
- Cheng, L.-W., Rao, S., Poudyal, S., Wang, P.-C., & Chen, S.-C. (2021). Genotype and virulence gene analyses of Bacillus cereus group clinical isolates from the Chinese softshell turtle (Pelodiscus sinensis) in Taiwan. Journal of Fish Diseases, 44, 1515– 1529. https://doi.org/10.1111/jfd.13473
- Gillis, Annika; Mahillon, Jacques (15 July 2014). "Prevalence, genetic diversity, and host range of tectiviruses among members of the Bacillus cereus group". Applied and Environmental Microbiology. 80 (14): 4138–4152. Bibcode:2014ApEnM..80.4138G. doi:10.1128/AEM.00912-14. ISSN 0099-2240. PMC 4068676. PMID 24795369.
External links
- Bacillus cereus genomes and related information at PATRIC, a Bioinformatics Resource Center funded by NIAID
- Type strain of Bacillus cereus at BacDive – the Bacterial Diversity Metadatabase