Corynebacterium striatum
Corynebacterium striatum is a bacterium that is a member of the Corynebacterium genus.[1] It is classified as non-diphtheritic.[2] The bacterium is a gram-positive prokaryote that assumes a 'club-like' morphology, more formally known as a corynebacteria structure.[1][3][4] It is non-lipophilic and undergoes aerobic respiration and is also a facultative anaerobe it is catalase negative and oxidase positive glucose and sucrose fermenter.[1][3]
| Corynebacterium striatum | |
|---|---|
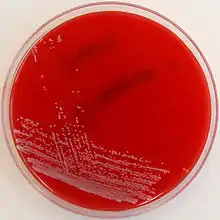 | |
| Corynebacterium striatum on Columbia Horse Blood Agar (HBA) | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Actinomycetota |
| Class: | Actinomycetia |
| Order: | Mycobacteriales |
| Family: | Corynebacteriaceae |
| Genus: | Corynebacterium |
| Species: | C. striatum |
| Binomial name | |
| Corynebacterium striatum (Chester 1901) Eberson 1918 (Approved Lists 1980) | |
It is generally found as a ubiquitous microorganism, and, as a commensal of humans, colonising the nasopharynx.[1][5] It has recently been recognised as an emerging pathogen although the genus of Corynebacterium is not usually considered to be pathogenic. Particularly in the context of human disease, Corynebacterium striatum is generally considered an opportunistic pathogenic, particularly in a nosocomial setting.[5][6] It has been recorded to infect the skin, upper and lower respiratory tract and even disseminate, resulting in sepsis. Recent interest has been sparked in the microorganism as it is known to be resistant to and gaining resistances to many antibiotics.[7]
History
Corynebacterium striatum is a member of the genus Corynebacterium.[4] Initially the species was described in 1901.[8] Scientific papers dating back until approximately 1980 recount cases of commensal Corynebacterium striatum contaminating samples from sites of infections. A paper published in 1993 found that isolates of described Corynebacterium Stratum stored by the American Type Culture Collection and the National Collection of Type Cultures were in fact not that of Corynebacterium striatum, although the recorded sequences corresponded with other known isolates of the species.[9] From this it was determined that at the time of isolation for storage, the incorrect bacteria was stored.[9] Up until 1993 there had only been three documented cases of respiratory infections caused by the species.[10] After this it was formally defined again in 1995.[6] Coryneform bacteria had for a long time been described as commensals of humans, colonising the skin and mucous membranes without causing disease.[11] More recently Corynebacterium striatum was found to in fact be the cause of infection and disease, given the opportunity.[6] Early clinical testing of hospital patients found that infection generally only occurred in immunocompromised individuals or those that had some for of prosthetic device permanently or intermittently fitted.[2] Not long after, researchers began to propose the notion that Corynebacterium striatum was the cause of disease even in patients that did not meet such criteria.[2][7]
Characteristics
Identification

Standardised methods of identification have been developed to improve the identification and isolation of Coronyforms. One such method is the API Coryne V2.0 system.[12] API strips combine a series of small scale biochemical tests to distinguish key characteristics of bacterium, based on metabolic activity.[13] The results are interpreted via standardised indicators and charts.[13] This is a cost-effective yet time-consuming method of identification, taking approximately 16 hours, particularly in clinical settings.
More modern identification techniques include genome sequencing – particularly 16S Ribosomal RNA (rRNA) Sequencing.[12] This technique relies on computerised comparison of genome sequences between bacterium.[14] The 16S rRNA sequence is highly conserved between bacterium, although will show slight variations and mutations between strains.[14] Comparison of variations in the Corynebacterium genome allow for specific identification of the Corynebacterium Striatum. It must be noted that currently this is currently a relatively expensive method of identification – when compared to the API strip, although this is improving as technologies improve.[14] The MALDI-TOF system is a clinically relevant method of detection that provides a rapid, 10 minute specific identification of bacteria.[12]
Both biochemical and molecular identification is accompanied by physical characterisation. Culturing the bacteria on blood cultures and gram staining to confirm morphology are integral additions to the process of identification.[11]
Physical
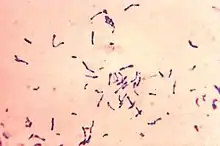
Corynebacterium striatum is a gram-positive bacterium, meaning they have a thin external peptidoglycan cell wall structure.[1][15] They have been described as having an irregular pleomorphic shape, and are non-motile.[16] Under a microscope they appearing as a hybrid of a bacillus and cocci morphology with a bulged pole attached to rod like end, more commonly described as a 'club-like' structure.[1][17] They are broadly described as being 1.5-8.0 micrometers in length, but formal measurement of individual clinical isolates will vary upon observation.[17] C. striatum colonies are able to be plated in vitro; when growing on Blood Agar the colonies will appear as small (1-2mm diameter) with a white, moist, and smooth appearance.[1][18] It is otherwise called a diphtheroid or coryneform due to its close phylogenetic relationship with diphtheria causing bacterium Corynebacterium diphtheriae.[4]
Molecular/biochemical
Corynebacterium striatum can be differentiated from other Corynebacterium types based on its ability to ferment glucose and sucrose, and inability to ferment maltose.[1][16] In comparison to other members of the genus Corynebacterium, it ferments sugars rapidly.[5] Corynebacterium species are also known to ferment nitrates.[16] It is also able to hydrolyse Tyrosine and Pyrazinamide. It does not metabolize urease, can hydrolyze esculin, and can also ferment mannitol and xylose.[5] In a laboratory setting, it is best distinguished from other coronyforms through its fermentative activity. They are non-lipophilic preferring to persist
Virulence factor
The organism itself possesses few virulence factors, giving it the title of an opportunistic colonizer as opposed to a true pathogen.[7] Virulence arises from its antibiotic resistance properties.[1] The increasing acquisition of antibiotic resistance genes allow for pathogenesis of Corynebacterium striatum colonisation.[1][6]
Antibiotic resistance
Antibiotic resistance is the acquisition of resistance to antibiotic treatments through either horizontal gene transfer or genetic mutation.[19] The acquisition of such characteristics by Corynebacterium striatum is relevant to its occurrence as a pathogen.
One study found that 93.7% of Corynebacterium striatum strains isolated showed resistance to at least one of the antimicrobial compounds tested. In this experiment, 82.5% of strains expressed resistance to the antibiotic penicillin.[7] Researchers deduced that due to the long-term exposure of Corynebacterium striatum to Penicillin resistance had been acquired by most isolates. In the same experiment, multi drug resistance was observed in 49.2% of strains.[7]
Corynebacterium striatum has been found to carry the bla gene.[7] This gene encodes a class A β-lactamase. β-lactamase are a group of antimicrobial enzymes that work to counter the effect of β-lactam antibiotics such as ampicillin and penicillin.
Further genes associated with antibiotic resistance include, but are not limited to: the gyrA gene which is attributed to resistance to the major antibiotic group fluoroquinolones, the aph(3′)-Ic gene imparting resistant to kanamycin, aph(3″)-Ib and aph(6)-Id causing resistant to streptomycin. Researchers also found that the erm(X) gene causes resistant to erythromycin[7][20][21]
Disease
Infection with Corynebacterium striatum was initially thought to occur through self-infection, transmitting the bacteria from a site where it persists as commensal and then allowing it to colonise in a situation as a pathogen.[2][22] It is now understood that it can be transmitted person to person, particularly in a hospital setting.[2][22]
While Corynebacterium infections are not common, when they have been observed it is in individuals with prosthetic devices or those who are immunosuppressed.[6] When detected under these conditions it is considered a true pathogen. Non-diphtheria Corynebacterium types are also being recognised for their impact on those suffering from long term respiratory disease.[23]
Infection
Many cases of infection with Corynebacterium striatum have been documented with particular relevance to acquisition of infection in hospitals – otherwise known as a nosocomial infection.[22][3] Corynebacterium striatum has been known to colonise prosthetics, particularly heart valves, prosthetic joints and even intravenous apparatus such as catheters.[7] Infections of this type have been described as a local infection or they can progress into a larger disseminated infection otherwise known as bacteraemia. Other documented infections include osteomyelitis, an infection of the bone that can occur through blood born infection or injury to the bone itself.[4][24]
One of the first described infections of an individual with Corynebacterium striatum occurred in 1980.[25] The man was severely immunocompromised, suffering already from leukemia.[25] After this, a number of isolated cases were documented. A well documented outbreak, at the Hospital Joan March, Mallorca, Spain, saw 21 individuals infected with Corynebacterium striatum.[22] The individuals all suffered from chronic obstructive pulmonary disease (COPD), and had also had significant tobacco exposure through their life, and as such were consistently being admitted to the hospital where they were treated by care staff with shared equipment.[22] In this instance Corynebacterium striatum was causing infection in the respiratory tract of patients and detected in sputum samples.[22] Of the 21 cases in the outbreak, there were six associated deaths.[22]
Another study of infection with Corynebacterium striatum described a patient who was admitted to hospital for treatment of cardiac arrest in 2008.[3] During her stay the patient was fitted with a central venous catheter through which she contracted bacteraemia of Corynebacterium striatum which resulted in her death.[3] The patient's age and immuno-compromised state resulting from pre-existing kidney failure ultimately allowed for the establishment and dissemination of infection.[3]
There is little to no evidence for Corynebacterium Striatum infection or colonising animal specimens.
Treatment
Multidrug resistance is the main factor considered when treating disease caused by Corynebacterium striatum.[19][6] Treatment with a mix of broad-spectrum antibiotics may thus be necessary. The selection of further antibiotic resistance is a serious consideration that must be made when selecting patient treatment. A study into the occurrence of non-diphtheroid infections determined that of all bacterium tested, Corynebacterium Striatum had the highest occurrence, accounting for 47% of infections.[26] In the same study, it was determined that all non-diphtheroid corynebacterium were susceptible to treatment with vancomycin, quinupristin-dalfopristin, linezolid and gentamicin.[26] While multiple studies confirmed that vancomycin was highly effective on all isolated species.[10][27] Previously it was known that Coronybacterium were susceptible to β-lactams, tetracycline, and fluoroquinolones, but recently, resistance genes to such treatments have been observed in clinical isolates.[27] Similar resistance was noted in a study with all isolates showing resistance to ciprofloxacin.[10] Of significant clinical significance is a rising resistance to beta-lactams in the last two decades, a group of antibiotics that includes penicillin.[28] Suggested oral treatments include a class of oxazolidinones - linezolid.[27] Although treatment with linezolid is not often prescribed as sustained treatment can affect liver function and have negative side effects including headaches and nausea.[29] Although differing results of susceptibility to antibiotic treatment has been noted between experiments, as such specific susceptibility of isolates should be obtained through antibiotic sensitivity testing. In clinical situations the antibiotic sensitivity can be obtained through disk diffusion assays of E-strip test. Treatment of clinical cases, such as the outbreak at the Hospital Joan March, were treated case by case, based on the antibiogram obtained from each patient's sputum sample.[22]
More broadly, prevention of initial infection, particularly in a hospital setting, is a key element of treatment. Sterilising all surfaces, and prostheses, as well as constant replacement and maintenance of prosthesis, is an integral element of stopping disease establishment.[30]
References
- Funke G, von Graevenitz A, Clarridge JE, Bernard KA (January 1997). "Clinical microbiology of coryneform bacteria". Clinical Microbiology Reviews. 10 (1): 125–59. doi:10.1128/CMR.10.1.125. PMC 172946. PMID 8993861.
- Chen FL, Hsueh PR, Teng SO, Ou TY, Lee WS (June 2012). "Corynebacterium striatum bacteremia associated with central venous catheter infection". Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi. 45 (3): 255–8. doi:10.1016/j.jmii.2011.09.016. PMID 22154992.
- Martín MC, Melón O, Celada MM, Alvarez J, Méndez FJ, Vázquez F (July 2003). "Septicaemia due to Corynebacterium striatum: molecular confirmation of entry via the skin". Journal of Medical Microbiology. 52 (Pt 7): 599–602. doi:10.1099/jmm.0.05102-0. PMID 12808083.
- Bernard K (October 2012). "The genus corynebacterium and other medically relevant coryneform-like bacteria". Journal of Clinical Microbiology. 50 (10): 3152–8. doi:10.1128/JCM.00796-12. PMC 3457441. PMID 22837327.
- Martínez-Martínez L, Suárez AI, Rodríguez-Baño J, Bernard K, Muniáin MA (February 1997). "Clinical significance of Corynebacterium striatum isolated from human samples". Clinical Microbiology and Infection. 3 (6): 634–639. doi:10.1111/j.1469-0691.1997.tb00470.x. PMID 11864205.
- Watkins DA, Chahine A, Creger RJ, Jacobs MR, Lazarus HM (July 1993). "Corynebacterium striatum: a diphtheroid with pathogenic potential". Clinical Infectious Diseases. 17 (1): 21–5. doi:10.1093/clinids/17.1.21. PMID 8353242.
- Alibi S, Ferjani A, Boukadida J, Cano ME, Fernández-Martínez M, Martínez-Martínez L, Navas J (August 2017). "Occurrence of Corynebacterium striatum as an emerging antibiotic-resistant nosocomial pathogen in a Tunisian hospital". Scientific Reports. 7 (1): 9704. Bibcode:2017NatSR...7.9704A. doi:10.1038/s41598-017-10081-y. PMC 5573724. PMID 28848236.
- Bergey DH, Breed RS (1957). Bergey's manual of determinative bacteriology (7th. ed.). Baltimore: Williams & Wilkins Co.
- Coyle MB, Leonard RB, Nowowiejski DJ (October 1993). "Pursuit of the Corynebacterium striatum type strain". International Journal of Systematic Bacteriology. 43 (4): 848–51. doi:10.1099/00207713-43-4-848. PMID 8240967.
- Renom F, Gomila M, Garau M, Gallegos MD, Guerrero D, Lalucat J, Soriano JB (July 2014). "Respiratory infection by Corynebacterium striatum: epidemiological and clinical determinants". New Microbes and New Infections. 2 (4): 106–14. doi:10.1002/nmi2.48. PMC 4184579. PMID 25356355.
- Daisuke U, Oishi T, Yamane K, Terada K (2017). "Corynebacterium striatum Bacteremia Associated with a Catheter-Related Blood Stream Infection". Case Reports in Infectious Diseases. 2017: 2682149. doi:10.1155/2017/2682149. PMC 5286468. PMID 28197349.
- Vila J, Juiz P, Salas C, Almela M, de la Fuente CG, Zboromyrska Y, Navas J, Bosch J, Agüero J, de la Bellacasa JP, Martínez-Martínez L (May 2012). "Identification of clinically relevant Corynebacterium spp., Arcanobacterium haemolyticum, and Rhodococcus equi by matrix-assisted laser desorption ionization-time of flight mass spectrometry". Journal of Clinical Microbiology. 50 (5): 1745–7. doi:10.1128/JCM.05821-11. PMC 3347156. PMID 22337985.
- "API®". bioMérieux. Retrieved 2019-05-26.
- Janda JM, Abbott SL (September 2007). "16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls". Journal of Clinical Microbiology. 45 (9): 2761–4. doi:10.1128/JCM.01228-07. PMC 2045242. PMID 17626177.
- "Gram Staining". Microscopy. Retrieved 2019-05-12.
- Martínez-Martínez L, Suárez AI, Winstanley J, Ortega MC, Bernard K (September 1995). "Phenotypic characteristics of 31 strains of Corynebacterium striatum isolated from clinical samples". Journal of Clinical Microbiology. 33 (9): 2458–61. doi:10.1128/JCM.33.9.2458-2461.1995. PMC 228438. PMID 7494046.
- "Description of the Bacterial Genus - Corynebacterium". catalog.hardydiagnostics.com. Retrieved 2019-05-26.
- tankeshwar (2013-09-08). "Colony Morphology of Bacteria; How to describe Bacterial Colonies?". Microbeonline. Retrieved 2019-05-26.
- Nikaido H (2009). "Multidrug resistance in bacteria". Annual Review of Biochemistry. 78: 119–46. doi:10.1146/annurev.biochem.78.082907.145923. PMC 2839888. PMID 19231985.
- Nie L, Lv Y, Yuan M, Hu X, Nie T, Yang X, Li G, Pang J, Zhang J, Li C, Wang X, You X (August 2014). "Genetic basis of high level aminoglycoside resistance in Acinetobacter baumannii from Beijing, China". Acta Pharmaceutica Sinica B. 4 (4): 295–300. doi:10.1016/j.apsb.2014.06.004. PMC 4629078. PMID 26579398.
- Ortiz-Pérez A, Martín-de-Hijas NZ, Esteban J, Fernández-Natal MI, García-Cía JI, Fernández-Roblas R (December 2010). "High frequency of macrolide resistance mechanisms in clinical isolates of Corynebacterium species". Microbial Drug Resistance. 16 (4): 273–7. doi:10.1089/mdr.2010.0032. PMID 20624090. S2CID 31356982.
- Renom F, Garau M, Rubí M, Ramis F, Galmés A, Soriano JB (June 2007). "Nosocomial outbreak of Corynebacterium striatum infection in patients with chronic obstructive pulmonary disease". Journal of Clinical Microbiology. 45 (6): 2064–7. doi:10.1128/JCM.00152-07. PMC 1933039. PMID 17409213.
- Díez-Aguilar M, Ruiz-Garbajosa P, Fernández-Olmos A, Guisado P, Del Campo R, Quereda C, Cantón R, Meseguer MA (June 2013). "Non-diphtheriae Corynebacterium species: an emerging respiratory pathogen". European Journal of Clinical Microbiology & Infectious Diseases. 32 (6): 769–72. doi:10.1007/s10096-012-1805-5. PMID 23271676. S2CID 17851143.
- "Osteomyelitis". Department of Health & Human Services. State Government of Victoria, Australia. Retrieved 2019-05-26.
- Bowstead, Thomas T.; Santiago, Silverio M. (January 1980). "Pleuropulmonary infection due to Corynebacterium striatum". British Journal of Diseases of the Chest. 74 (2): 198–200. doi:10.1016/0007-0971(80)90035-2. ISSN 0007-0971. PMID 7426360.
- Neemuchwala A, Soares D, Ravirajan V, Marchand-Austin A, Kus JV, Patel SN (April 2018). "In Vitro Antibiotic Susceptibility Pattern of Non-diphtheriae Corynebacterium Isolates in Ontario, Canada, from 2011 to 2016". Antimicrobial Agents and Chemotherapy. 62 (4): e01776–17. doi:10.1128/AAC.01776-17. PMC 5914010. PMID 29339389.
- Hahn WO, Werth BJ, Butler-Wu SM, Rakita RM (November 2016). "Multidrug-Resistant Corynebacterium striatum Associated with Increased Use of Parenteral Antimicrobial Drugs". Emerging Infectious Diseases. 22 (11). doi:10.3201/eid2211.160141. PMC 5088002. PMID 27767926.
- Funke G, Pünter V, von Graevenitz A (December 1996). "Antimicrobial susceptibility patterns of some recently established coryneform bacteria". Antimicrobial Agents and Chemotherapy. 40 (12): 2874–8. doi:10.1128/AAC.40.12.2874. PMC 163638. PMID 9124857.
- "Linezolid". NPS MedicineWise. Retrieved 2019-05-26.
- "Basics on Processing & Sterilization". Sterile & Materials Processing Department. University of Rochester Medical Center. Retrieved 2019-05-13.