Hypervitaminosis A
Hypervitaminosis A refers to the toxic effects of ingesting too much preformed vitamin A (retinyl esters, retinol, and retinal). Symptoms arise as a result of altered bone metabolism and altered metabolism of other fat-soluble vitamins. Hypervitaminosis A is believed to have occurred in early humans, and the problem has persisted throughout human history. Toxicity results from ingesting too much preformed vitamin A from foods (such as fish liver or animal liver), supplements, or prescription medications and can be prevented by ingesting no more than the recommended daily amount.
| Hypervitaminosis A | |
|---|---|
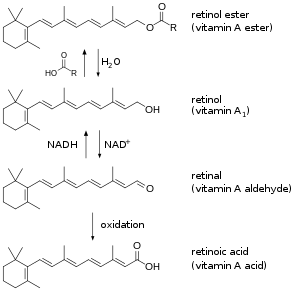 | |
| Forms of preformed vitamin A in the body | |
| Specialty | Toxicology |
Diagnosis can be difficult, as serum retinol is not sensitive to toxic levels of vitamin A, but there are effective tests available. Hypervitaminosis A is usually treated by stopping intake of the offending food(s), supplement(s), or medication. Most people make a full recovery. High intake of provitamin carotenoids (such as beta-carotene) from vegetables and fruits does not cause hypervitaminosis A.
Signs and symptoms
Symptoms may include:
- Changes in consciousness
- Decreased appetite
- Dizziness
- Vision changes, double vision (young children)
- Drowsiness
- Headache
- Irritability
- Nausea
- Poor weight gain (infants and children)
- Skin and hair changes
- Cracking at corners of the mouth
- Hair loss
- Higher sensitivity to sunlight
- Oily skin and hair (seborrhea)
- Skin peeling, itching
- Vomiting
- Yellow discoloration of the skin (aurantiasis cutis)
Signs
- Abnormal softening of the skull bone (craniotabes—infants and children)
- Blurred vision
- Bone pain or swelling
- Bulging fontanelle (infants)
- Gastric mucosal calcinosis[1]
- Heart valve calcification[2]
- Hypercalcemia
- Increased intracranial pressure manifesting as cerebral edema, papilledema, and headache[3] (may be referred to as Idiopathic intracranial hypertension)
- Liver damage[4][5][6][7][8][9][10][11][12]
- Premature epiphyseal closure[13][14][15][16][17]
- Spontaneous fracture[18][19]
- Uremic pruritus[20]
Causes
.JPG.webp)
Hypervitaminosis A results from excessive intake of preformed vitamin A. Genetic variations in tolerance to vitamin A intake may occur, so the toxic dose will not be the same for everyone.[21] Children are particularly sensitive to vitamin A, with daily intakes of 1500 IU/kg body weight reportedly leading to toxicity.[19]
Types of vitamin A
- It is "largely impossible" for provitamin carotenoids, such as beta-carotene, to cause toxicity, as their conversion to retinol is highly regulated.[19] No vitamin A toxicity has ever been reported from ingestion of excessive amounts.[22] Overconsumption of beta-carotene can only cause carotenosis, a harmless and reversible cosmetic condition in which the skin turns orange.
- Preformed vitamin A absorption and storage in the liver occur very efficiently until a pathologic condition develops.[19] When ingested, 70–90% of preformed vitamin A is absorbed and used.[19]
Sources of toxicity
- Diet – Liver is high in vitamin A. The liver of certain animals, including the polar bear, bearded seal,[23][24] fish,[25] walrus,[26] and moose,[27] are particularly toxic (see Liver (food) § Poisoning). It has been estimated that consumption of 500 grams of polar bear liver would result in a toxic dose for a human.[23]
- Supplements – Dietary supplements can be toxic when taken above recommended dosages.
Types of toxicity
- Acute toxicity occurs over a period of hours or a few days, and is less of a problem than chronic toxicity.
- Chronic toxicity results from daily intakes greater than 25,000 IU for 6 years or longer and more than 100,000 IU for 6 months or longer.
Mechanism
Absorption and storage in the liver of retinol occur very efficiently until a pathologic condition develops.[19]
Absorption
When ingested, 70–90% of preformed vitamin A is absorbed and used.[19]
According to a 2003 review, water-miscible, emulsified, and solid forms of vitamin A supplements are more toxic than oil-based supplement and liver sources.[28]
Storage
80–90% of the total body reserves of preformed vitamin A are in the liver (with 80–90% of this amount being stored in hepatic stellate cells and the remaining 10–20% being stored in hepatocytes). Fat is another significant storage site, while the lungs and kidneys may also be capable of storage.[19]
Transport
Until recently, it was thought that the sole important retinoid delivery pathway to tissues involved retinol bound to retinol-binding protein (RBP4). More recent findings, however, indicate that retinoids can be delivered to tissues through multiple overlapping delivery pathways, involving chylomicrons, very low density lipoprotein (VLDL) and low density lipoprotein (LDL), retinoic acid bound to albumin, water-soluble β-glucuronides of retinol and retinoic acid, and provitamin A carotenoids.[29]
The range of serum retinol concentrations under normal conditions is 1–3 μmol/L. Elevated amounts of retinyl ester (i.e., >10% of total circulating vitamin A) in the fasting state have been used as markers for chronic hypervitaminosis A in humans. Candidate mechanisms for this increase include decreased hepatic uptake of vitamin A and the leaking of esters into the bloodstream from saturated hepatic stellate cells.[19]
Effects
Effects include increased bone turnover and altered metabolism of fat-soluble vitamins. More research is needed to fully elucidate the effects.
Increased bone turnover
Retinoic acid suppresses osteoblast activity and stimulates osteoclast formation in vitro,[22] resulting in increased bone resorption and decreased bone formation. It is likely to exert this effect by binding to specific nuclear receptors (members of the retinoic acid receptor or retinoid X receptor nuclear transcription family) which are found in every cell (including osteoblasts and osteoclasts).
This change in bone turnover is likely to be the reason for numerous effects seen in hypervitaminosis A, such as hypercalcemia and numerous bone changes such as bone loss that potentially leads to osteoporosis, spontaneous bone fractures, altered skeletal development in children, skeletal pain, radiographic changes,[19][22] and bone lesions.[30]
Altered fat-soluble vitamin metabolism
Preformed vitamin A is fat-soluble and high levels have been reported to affect metabolism of the other fat-soluble vitamins D,[22] E, and K.
The toxic effects of preformed vitamin A might be related to altered vitamin D metabolism, concurrent ingestion of substantial amounts of vitamin D, or binding of vitamin A to receptor heterodimers. Antagonistic and synergistic interactions between these two vitamins have been reported, as they relate to skeletal health.
Stimulation of bone resorption by vitamin A has been reported to be independent of its effects on vitamin D.[22]
Mitochondrial toxicity
Preformed vitamin A and retinoids exerts several toxic effects regarding redox environment and mitochondrial function. [31]
Diagnosis
Retinol concentrations are nonsensitive indicators
Assessing vitamin A status in persons with subtoxicity or toxicity is complicated because serum retinol concentrations are not sensitive indicators in this range of liver vitamin A reserves.[19] The range of serum retinol concentrations under normal conditions is 1–3 μmol/L and, because of homeostatic regulation, that range varies little with widely disparate vitamin A intakes.[19]
Retinol esters have been used as markers
Retinyl esters can be distinguished from retinol in serum and other tissues and quantified with the use of methods such as high-performance liquid chromatography.[19]
Elevated amounts of retinyl ester (i.e., >10% of total circulating vitamin A) in the fasting state have been used as markers for chronic hypervitaminosis A in humans and monkeys.[19] This increased retinyl ester may be due to decreased hepatic uptake of vitamin A and the leaking of esters into the bloodstream from saturated hepatic stellate cells.[19]
Prevention
Hypervitaminosis A can be prevented by not ingesting more than the US Institute of Medicine Daily Tolerable Upper Level of intake for Vitamin A. This level is for synthetic and natural retinol ester forms of vitamin A. Carotene forms from dietary sources are not toxic. Possible pregnancy, liver disease, high alcohol consumption, and smoking are indications for close monitoring and limitation of vitamin A administration.
Daily tolerable upper level
| Life stage group category | Upper Level (μg/day) |
|---|---|
| Infants
0–6 months |
600 600 |
| Children
1–3 years |
600 900 |
| Males
9–13 years |
1700 2800 3000 |
| Females
9–13 years |
1700 2800 3000 |
| Pregnancy
<19 years |
2800 3000 |
| Lactation
<19 years |
2800 3000 |
Treatment
- Stopping high vitamin A intake is the standard treatment. Most people fully recover.
- Phosphatidylcholine (in the form of PPC or DLPC), the substrate for Lecithin retinol acyltransferase, which converts retinol into Retinyl esters (the storage forms of vitamin A).
- Vitamin E may alleviate hypervitaminosis A.[32]
- Liver transplantation may be a valid option if no improvement occurs.[33]
If liver damage has progressed into fibrosis, synthesizing capacity is compromised and supplementation can replenish PC. However, recovery is dependent on removing the causative agent: halting high Vitamin A intake.[34][35][36][37]
History
Vitamin A toxicity is known to be an ancient phenomenon; fossilized skeletal remains of early humans suggest bone abnormalities may have been caused by hypervitaminosis A.[19] There are two theories for hypervitaminosis A in the isolated case of KMN-ER 1808. One of these is the increased consumption of meat and the second is an increase in insect consumption, or Entomophagy.
Vitamin A toxicity has long been known to the Inuit as they will not eat the liver of polar bears or bearded seals due to them contain dangerous amounts of Vitamin A.[23] and has been known by Europeans since at least 1597 when Gerrit de Veer wrote in his diary that, while taking refuge in the winter in Nova Zemlya, he and his men became severely ill after eating polar bear liver.[38]
In 1913, Antarctic explorers Douglas Mawson and Xavier Mertz were both poisoned (and Mertz died) from eating the livers of their sled dogs during the Far Eastern Party.[39] Another study suggests, however, that exhaustion and diet change are more likely to have caused the tragedy.[40]
Other animals
Some Arctic animals demonstrate no signs of hypervitaminosis A despite having 10–20 times the level of vitamin A in their livers as other Arctic animals. These animals are top predators and include the polar bear, Arctic fox, bearded seal, and glaucous gull. This ability to efficiently store higher amounts of vitamin A may have contributed to their survival in the extreme environment of the Arctic.[41]
Treatment
These treatments have been used to help treat or manage toxicity in animals. Although not considered part of standard treatment, they might be of some benefit to humans.
- Vitamin E appears to be an effective treatment in rabbits, and[42] prevents side effects in chicks[43]
- Taurine significantly reduces toxic effects in rats.[44] Retinoids can be conjugated by taurine and other substances. Significant amounts of retinotaurine are excreted in the bile,[45] and this retinol conjugate is thought to be an excretory form, as it has little biological activity.[46]
- Red yeast rice ("cholestin") – significantly reduces toxic effects in rats.[47]
- Vitamin K prevents hypoprothrombinemia in rats and can sometimes control the increase in plasma/cell ratios of vitamin A.[48]
See also
- Vitamin poisoning
- Far Eastern Party
- Retinoic acid syndrome
- Piblokto
References
- Gorospe M, Fadare O (May 2007). "Gastric mucosal calcinosis: clinicopathologic considerations". Advances in Anatomic Pathology. 14 (3): 224–8. doi:10.1097/PAP.0b013e31805048ea. PMID 17452819. S2CID 45905601.
- Huk DJ, Hammond HL, Kegechika H, Lincoln J (February 2013). "Increased dietary intake of vitamin A promotes aortic valve calcification in vivo". Arteriosclerosis, Thrombosis, and Vascular Biology. 33 (2): 285–93. doi:10.1161/ATVBAHA.112.300388. PMC 3557503. PMID 23202364.
- Wall M (March 2008). "Idiopathic intracranial hypertension (pseudotumor cerebri)". Current Neurology and Neuroscience Reports. 8 (2): 87–93. doi:10.1007/s11910-008-0015-0. PMID 18460275. S2CID 17285706.
- Castaño G, Etchart C, Sookoian S (2006). "Vitamin A toxicity in a physical culturist patient: a case report and review of the literature". Annals of Hepatology. 5 (4): 293–395. doi:10.1016/S1665-2681(19)31992-1. PMID 17151585.
- Minuk GY, Kelly JK, Hwang WS (1988). "Vitamin A hepatotoxicity in multiple family members". Hepatology. 8 (2): 272–5. doi:10.1002/hep.1840080214. PMID 3356407. S2CID 6632550.
- Levine PH, Delgado Y, Theise ND, West AB (February 2003). "Stellate-cell lipidosis in liver biopsy specimens. Recognition and significance". American Journal of Clinical Pathology. 119 (2): 254–8. doi:10.1309/6DKC-03C4-GAAE-N2DK. PMID 12579996.
- Tholen W, Paquet KJ, Rohner HG, Albrecht M (August 1980). "[Cirrhosis of the liver and esophageal bleeding after chronic vitamin A intoxication (author's transl)]". Leber, Magen, Darm. 10 (4): 193–7. PMID 6969836.
- Jorens PG, Michielsen PP, Pelckmans PA, Fevery J, Desmet VJ, Geubel AP, Rahier J, Van Maercke YM (December 1992). "Vitamin A abuse: development of cirrhosis despite cessation of vitamin A. A six-year clinical and histopathologic follow-up". Liver. 12 (6): 381–6. doi:10.1111/j.1600-0676.1992.tb00592.x. PMID 1470008.
- Babb RR, Kieraldo JH (March 1978). "Cirrhosis due to hypervitaminosis A". The Western Journal of Medicine. 128 (3): 244–6. PMC 1238074. PMID 636413.
- Erickson JM, Mawson AR (September 2000). "Possible role of endogenous retinoid (Vitamin A) toxicity in the pathophysiology of primary biliary cirrhosis". Journal of Theoretical Biology. 206 (1): 47–54. Bibcode:2000JThBi.206...47E. doi:10.1006/jtbi.2000.2102. PMID 10968936.
- Singh M, Singh VN (May 1978). "Fatty liver in hypervitaminosis A: synthesis and release of hepatic triglycerides". The American Journal of Physiology. 234 (5): E511–4. doi:10.1152/ajpendo.1978.234.5.E511. PMID 645903.
- Nollevaux MC, Guiot Y, Horsmans Y, Leclercq I, Rahier J, Geubel AP, Sempoux C (March 2006). "Hypervitaminosis A-induced liver fibrosis: stellate cell activation and daily dose consumption". Liver International. 26 (2): 182–6. doi:10.1111/j.1478-3231.2005.01207.x. PMID 16448456. S2CID 41658180.
- Cho DY, Frey RA, Guffy MM, Leipold HW (November 1975). "Hypervitaminosis A in the dog". American Journal of Veterinary Research. 36 (11): 1597–1603. PMID 1190603.
- Kodaka T, Takaki H, Soeta S, Mori R, Naito Y (July 1998). "Local disappearance of epiphyseal growth plates in rats with hypervitaminosis A". The Journal of Veterinary Medical Science. 60 (7): 815–21. doi:10.1292/jvms.60.815. PMID 9713809.
- Soeta S, Mori R, Kodaka T, Naito Y, Taniguchi K (March 1999). "Immunohistochemical observations on the initial disorders of the epiphyseal growth plate in rats induced by high dose of vitamin A". The Journal of Veterinary Medical Science. 61 (3): 233–8. doi:10.1292/jvms.61.233. PMID 10331194.
- Soeta S, Mori R, Kodaka T, Naito Y, Taniguchi K (March 2000). "Histological disorders related to the focal disappearance of the epiphyseal growth plate in rats induced by high dose of vitamin A". The Journal of Veterinary Medical Science. 62 (3): 293–9. doi:10.1292/jvms.62.293. PMID 10770602.
- Rothenberg AB, Berdon WE, Woodard JC, Cowles RA (December 2007). "Hypervitaminosis A-induced premature closure of epiphyses (physeal obliteration) in humans and calves (hyena disease): a historical review of the human and veterinary literature". Pediatric Radiology. 37 (12): 1264–7. doi:10.1007/s00247-007-0604-0. PMID 17909784. S2CID 34194762.
- Wick JY (February 2009). "Spontaneous fracture: multiple causes". The Consultant Pharmacist. 24 (2): 100–2, 105–8, 110–2. doi:10.4140/TCP.n.2009.100. PMID 19275452.
- Penniston KL, Tanumihardjo SA (February 2006). "The acute and chronic toxic effects of vitamin A". The American Journal of Clinical Nutrition. 83 (2): 191–201. doi:10.1093/ajcn/83.2.191. PMID 16469975.
- Corić-Martinović V, Basić-Jukić N (2008). "[Uremic pruritus]". Acta Medica Croatica. 62 Suppl 1: 32–6. PMID 18578330.
- Carpenter TO, Pettifor JM, Russell RM, Pitha J, Mobarhan S, Ossip MS, Wainer S, Anast CS (October 1987). "Severe hypervitaminosis A in siblings: evidence of variable tolerance to retinol intake". The Journal of Pediatrics. 111 (4): 507–12. doi:10.1016/s0022-3476(87)80109-9. PMID 3655980.
- Barker ME, Blumsohn A (November 2003). "Is vitamin A consumption a risk factor for osteoporotic fracture?". The Proceedings of the Nutrition Society. 62 (4): 845–50. doi:10.1079/PNS2003306. PMID 15018484.
- Rodahl K, Moore T (July 1943). "The vitamin A content and toxicity of bear and seal liver". The Biochemical Journal. 37 (2): 166–8. doi:10.1042/bj0370166. PMC 1257872. PMID 16747610.
- The Phoca barbata listed on pages 167–168 of the previous reference is now known as Erignathus barbatus
- Schmitt C, Domangé B, Torrents R, de Haro L, Simon N (December 2020). "Hypervitaminosis A Following the Ingestion of Fish Liver: Report on 3 Cases from the Poison Control Center in Marseille". Wilderness Environ Med. 31 (4): 454–456. doi:10.1016/j.wem.2020.06.003. PMID 32861618.
- "Walrus, liver, raw (Alaska Native)". Mealographer. Retrieved 2010-03-25.
- "Moose, liver, braised (Alaska Native)". Mealographer. Retrieved 2012-10-15.
- Myhre, Anne M; Monica H Carlsen; Siv K Bøhn; Heidi L Wold; Petter Laake; Rune Blomhoff (2003-12-01). "Water-Miscible, Emulsified, and Solid Forms of Retinol Supplements Are More Toxic Than Oil-Based Preparations". The American Journal of Clinical Nutrition. 78 (6): 1152–1159. doi:10.1093/ajcn/78.6.1152. ISSN 0002-9165. PMID 14668278. Retrieved 2012-04-16.
- Li Y, Wongsiriroj N, Blaner WS (June 2014). "The multifaceted nature of retinoid transport and metabolism". Hepatobiliary Surgery and Nutrition. 3 (3): 126–39. doi:10.3978/j.issn.2304-3881.2014.05.04. PMC 4073323. PMID 25019074.
- Hough S, Avioli LV, Muir H, Gelderblom D, Jenkins G, Kurasi H, Slatopolsky E, Bergfeld MA, Teitelbaum SL (June 1988). "Effects of hypervitaminosis A on the bone and mineral metabolism of the rat". Endocrinology. 122 (6): 2933–9. doi:10.1210/endo-122-6-2933. PMID 3371268.
- de Oliveira MR (2015). "Vitamin A and Retinoids as Mitochondrial Toxicants". Oxidative Medicine and Cellular Longevity. 2015: 1–13. doi:10.1155/2015/140267. PMC 4452429. PMID 26078802.
- McCuaig LW, Motzok I (July 1970). "Excessive dietary vitamin E: its alleviation of hypervitaminosis A and lack of toxicity". Poultry Science. 49 (4): 1050–1. doi:10.3382/ps.0491050. PMID 5485475.
- Cheruvattath R, Orrego M, Gautam M, Byrne T, Alam S, Voltchenok M, Edwin M, Wilkens J, Williams JW, Vargas HE (December 2006). "Vitamin A toxicity: when one a day doesn't keep the doctor away". Liver Transplantation. 12 (12): 1888–91. doi:10.1002/lt.21007. PMID 17133567. S2CID 32290718.
- Gundermann KJ, Kuenker A, Kuntz E, Droździk M (2011). "Activity of essential phospholipids (EPL) from soybean in liver diseases". Pharmacological Reports. 63 (3): 643–59. doi:10.1016/S1734-1140(11)70576-X. PMID 21857075.
- Okiyama W, Tanaka N, Nakajima T, Tanaka E, Kiyosawa K, Gonzalez FJ, Aoyama T (June 2009). "Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress". Journal of Hepatology. 50 (6): 1236–46. doi:10.1016/j.jhep.2009.01.025. PMC 2809859. PMID 19398233.
- Wu J, Zern MA (2000). "Hepatic stellate cells: a target for the treatment of liver fibrosis". Journal of Gastroenterology. 35 (9): 665–72. doi:10.1007/s005350070045. PMID 11023037. S2CID 40851639.
- Navder KP, Lieber CS (March 2002). "Dilinoleoylphosphatidylcholine is responsible for the beneficial effects of polyenylphosphatidylcholine on ethanol-induced mitochondrial injury in rats". Biochemical and Biophysical Research Communications. 291 (4): 1109–12. doi:10.1006/bbrc.2002.6557. PMID 11866479.
- Lips P (January 2003). "Hypervitaminosis A and fractures". The New England Journal of Medicine. 348 (4): 347–9. doi:10.1056/NEJMe020167. PMID 12540650.
- Nataraja, Anjali (2002). "Man's best friend?". Student BMJ. 10: 131–70.
- Carrington-Smith D (2005). "Mawson and Mertz: a re-evaluation of their ill-fated mapping journey during the 1911-1914 Australasian Antarctic Expedition". The Medical Journal of Australia. 183 (11–12): 638–41. doi:10.5694/j.1326-5377.2005.tb00064.x. PMID 16336159. S2CID 8430414.
- Senoo H, Imai K, Mezaki Y, Miura M, Morii M, Fujiwara M, Blomhoff R (October 2012). "Accumulation of vitamin A in the hepatic stellate cell of arctic top predators". Anatomical Record. 295 (10): 1660–8. doi:10.1002/ar.22555. PMID 22907891.
- St Claire MB, Kennett MJ, Besch-Williford CL (July 2004). "Vitamin A toxicity and vitamin E deficiency in a rabbit colony". Contemporary Topics in Laboratory Animal Science. 43 (4): 26–30. PMID 15264766.
- Weiser H, Probst HP, Bachmann H (September 1992). "Vitamin E prevents side effects of high doses of vitamin A in chicks". Annals of the New York Academy of Sciences. 669 (1): 396–8. Bibcode:1992NYASA.669..396W. doi:10.1111/j.1749-6632.1992.tb17134.x. PMID 1444058. S2CID 40860314.
- Yeh Y, Lee Y, Hsieh H, Hwang D (2008). "Effect of taurine on toxicity of vitamin a in rats". Food Chemistry. 106: 260–8. doi:10.1016/j.foodchem.2007.05.084.
- Skare KL, DeLuca HF (July 1983). "Biliary metabolites of all-trans-retinoic acid in the rat". Archives of Biochemistry and Biophysics. 224 (1): 13–8. doi:10.1016/0003-9861(83)90185-6. PMID 6870249.
- Skare KL, Sietsema WK, DeLuca HF (August 1982). "The biological activity of retinotaurine". The Journal of Nutrition. 112 (8): 1626–30. doi:10.1093/jn/112.8.1626. PMID 7097369.
- Yeh YH, Lee YT, Hsieh YL (May 2012). "Effect of cholestin on toxicity of vitamin A in rats". Food Chemistry. 132 (1): 311–8. doi:10.1016/j.foodchem.2011.10.082. PMID 26434295.
- Walker SE, Eylenburg E, Moore T (1947). "The action of vitamin K in hypervitaminosis A". The Biochemical Journal. 41 (4): 575–80. doi:10.1042/bj0410575. PMC 1258540. PMID 16748217.
External links
- Facts about Vitamin A and Carotenoids, from the National Institutes of Health's Office of Dietary Supplements.