Lilotomab
Lilotomab (formerly tetulomab, HH1)[1] is a murine monoclonal antibody against CD37,[2] a glycoprotein which is expressed on the surface of mature human B cells.[3] It was generated at the Norwegian Radium Hospital.[3]
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Mouse |
| Target | CD37 |
| Clinical data | |
| Other names | Tetulomab, HH1 |
| ATC code |
|
| Identifiers | |
| CAS Number |
|
| ChemSpider |
|
| UNII | |
| KEGG | |
As of 2016 it was under development by the Norwegian company Nordic Nanovector ASA as a radioimmunotherapeutic in which lilotomab is conjugated to the beta radiation-emitting isotope lutetium-177 by means of a linker called satetraxetan, a derivative of DOTA.[2] This compound is called 177Lu-HH1 or lutetium (177Lu) lilotomab satetraxetan (trade name Betalutin).[1] As of 2016, a phase 1/2 clinical trial in people with non-Hodgkin lymphoma was underway.[4]
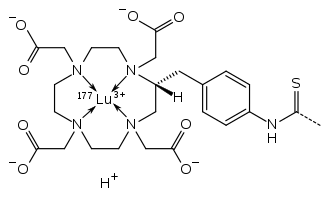
The satetraxetan structure chelating lutetium-177[5]
References
- "Lutetium (177lu) lilotomab satetraxetan - Nordic Nanovector". Adis Insight. Springer Nature Switzerland AG. Retrieved 1 September 2016.
- "Recommended INN List 74" (PDF). WHO Drug Information. 29 (3). 2015.
- Robak T, Robak P (May 2014). "Anti-CD37 antibodies for chronic lymphocytic leukemia". Expert Opinion on Biological Therapy. 14 (5): 651–61. doi:10.1517/14712598.2014.890182. PMID 24555705. S2CID 19987961.
- "EudraCT Number: 2011-000033-36". EU Clinical Trials Registry.
Treatment of lymphoma with targeted internal radiation therapy (Betalutin)
- "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 112" (PDF). WHO Drug Information. 28 (4): 515. 2014.
Further reading
- Repetto-Llamazares AH, Larsen RH, Mollatt C, Lassmann M, Dahle J (March 2013). "Biodistribution and dosimetry of (177)Lu-tetulomab, a new radioimmunoconjugate for treatment of non-Hodgkin lymphoma". Current Radiopharmaceuticals. 6 (1): 20–7. doi:10.2174/1874471011306010004. PMC 3624777. PMID 23256748.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.