Atezolizumab
Atezolizumab, sold under the brand name Tecentriq, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC), and hepatocellular carcinoma (HCC).[2] It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).[4]
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | PD-L1 |
| Clinical data | |
| Trade names | Tecentriq |
| Other names | MPDL3280A, RG7446 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a616035 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous infusion |
| Drug class | Antineoplastic agent |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6446H9902N1706O1998S42 |
| Molar mass | 144612.59 g·mol−1 |
The most common side effects when used on its own include tiredness, reduced appetite, nausea (feeling sick), vomiting, cough, difficulty breathing, diarrhea, rash, fever, pain in the back, joints, muscles and bones, weakness, itching and urinary tract infection (infection of the structures that carry urine).[3]
The most common side effects when used with other cancer medicines include peripheral neuropathy (nerve damage in the hands and feet), nausea, anaemia (low red blood cell counts), neutropenia (low white blood cell counts), thrombocytopenia (low platelet counts), rash, tiredness, constipation, reduced appetite, diarrhea, and cough.[3]
Atezolizumab is the first PD-L1 inhibitor approved by the U.S. Food and Drug Administration (FDA).[5]
Medical uses
In the European Union atezolizumab is indicated for the treatment of urothelial carcinoma, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), hepatocellular carcinoma, and breast cancer.[3][6]
In the United States, atezolizumab is indicated for the treatment of urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC), hepatocellular carcinoma (HCC), and melanoma.[2][7]
Adverse effects
The most common adverse effects in studies were fatigue, decreased appetite, nausea, and infections. Urinary tract infection was the most common severe adverse effect.[8]
Pharmacology
Mechanism of action
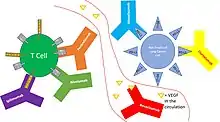
Atezolizumab blocks the interaction of PD-L1 with programmed cell death protein 1 (PD-1) and CD80 receptors (B7-1Rs).[10] PD-L1 can be highly expressed on certain tumors, which is thought to lead to reduced activation of immune cells (cytotoxic T-cells in particular) that might otherwise recognize and attack the cancer.[10] Inhibition of PD-L1 by atezolizumab can remove this inhibitor effect and thereby engender an anti-tumor response. It is one of several ways to block inhibitory signals related to T-cell activation, a more general strategy known as "immune checkpoint inhibition."[10]
For some cancers (notably bladder) the probability of benefit is related to PD-L1 expression, but most cancers with PD-L1 expression still do not respond, and many (about 15%) without PD-L1 expression do respond.[10]
History
In 2015, it was in clinical trials as an immunotherapy for several types of solid tumors.[4] It was under investigation by Genentech/Roche.[4]
In April 2016, Roche announced that atezolizumab had been granted fast track status for lung cancer by the U.S. Food and Drug Administration (FDA).[11]
In May 2016, the FDA granted accelerated approval to atezolizumab for locally advanced or metastatic urothelial carcinoma treatment after failure of cisplatin-based chemotherapy.[5][12] The confirmatory trial (to convert the accelerated approval into a full approval) failed to achieve its primary endpoint of overall survival.[13] In 2018, FDA altered the use of atezolizumab as a first-line treatment for metastatic bladder cancer in people who can't receive cisplatin-based chemotherapy and have high levels of PD-L1.[14]
In May 2016, atezolizumab was approved by the FDA for the treatment of people with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy or have disease progression within twelve months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy.[15][5] In May 2017, atezolizumab failed a phase III trial for second line bladder cancer.[16]
The safety and efficacy of atezolizumab were studied in people with urothelial carcinoma.[5][15][17] Tumors temporarily shrank in a minority of participants, with people being more likely to benefit if the tumor expressed PD-L1.[5] The trial was conducted in the United States, Canada, Spain, France, Great Britain, Germany, Italy and the Netherlands.[17]
In October 2016, atezolizumab was approved by the FDA for the treatment of people with metastatic non-small cell lung cancer (NSCLC) whose disease progressed during or following platinum-containing chemotherapy.[18] People with EGFR or ALK genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving atezolizumab.[18]
This approval was based on two international, randomized, open-label clinical trials (OAK and POPLAR) that demonstrated consistent results in efficacy and safety in a total of 1137 participants with NSCLC.[18] Compared with docetaxel, treatment with atezolizumab in the intended participants population in the two trials resulted in a 4.2 and a 2.9 month improvement in overall survival (OS), respectively.[18]
Atezolizumab was approved for medical use in the European Union in September 2017.[3]
In May 2018, atezolizumab used in combination with bevacizumab (Avastin) and standard chemotherapy for some people with lung cancer was granted priority review.[19]
In August 2018, the FDA updated the prescribing information for atezolizumab to require the use of an FDA-approved companion diagnostic test to determine PD-L1 levels in tumor tissue from people with locally advanced or metastatic urothelial cancer who are cisplatin-ineligible.[20]
In September 2018, it was announced that atezolizumab prolongs survival in extensive stage small cell lung cancer treatment, according to study results presented at the 19th World Conference on Lung Cancer (WCLC) in Toronto, Canada.
In October 2018, a combined clinical trial of the drug with nab-paclitaxel on people with advanced triple negative breast cancer concluded.[21]
Atezolizumab, in combination with bevacizumab, paclitaxel, and carboplatin, was approved in the United States in December 2018, for the first-line treatment of people with metastatic non-squamous, non-small cell lung cancer (NSq NSCLC) with no EGFR or ALK genomic tumor aberrations.[22] Approval was based on the IMpower150 trial (NCT02366143), an open-label, randomized (1:1:1), three-arm trial enrolling 1202 participants receiving first-line treatment for metastatic NSq NSCLC.[22]
In March 2019, it was approved in the United States, in combination with paclitaxel protein-bound, for adults with unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) whose tumors express PD-L1 (PD-L1 stained tumor-infiltrating immune cells [IC] of any intensity covering ≥ 1% of the tumor area), as determined by an FDA-approved test.[23] The FDA also approved the VENTANA PD-L1 (SP142) Assay as a companion diagnostic device for selecting TNBC patients for atezolizumab.[24]
Approval was based on IMpassion130 (NCT02425891), a multicenter, international, double-blinded, placebo-controlled, randomized trial that included 902 participants with unresectable locally advanced or metastatic TNBC who had not received prior chemotherapy for metastatic disease.[24] Participants were randomized (1:1) to receive either atezolizumab (840 mg) or placebo intravenous infusions on days 1 and 15 of every 28-day cycle, plus paclitaxel protein-bound (100 mg/m2) administered via intravenous infusion on days 1, 8, and 15 of every 28-day cycle.[24]
In March 2019, it was approved in the United States, in combination with carboplatin and etoposide, for the first-line treatment of adults with extensive-stage small cell lung cancer (ES-SCLC).[25]
Approval was based on IMpower133 (NCT02763579), a randomized (1:1), multicenter, double-blind, placebo-controlled trial in 403 participants with ES-SCLC who received no prior chemotherapy for extensive stage disease and had ECOG performance status 0 or 1.[25]
In December 2019, atezolizumab in combination with paclitaxel protein-bound and carboplatin was approved by the FDA for the first-line treatment of adults with metastatic non-squamous non-small cell lung cancer (NSCLC) with no EGFR or ALK genomic tumor aberrations.[26]
Efficacy was evaluated in IMpower130 (NCT02367781), a multicenter, randomized (2:1), open-label trial in participants with stage IV non-squamous NSCLC who had received no prior chemotherapy for metastatic disease, but could have received prior EGFR or ALK kinase inhibitor, if appropriate.[26] The trial randomized 724 participants (ITT) to receive atezolizumab, paclitaxel protein-bound, and carboplatin, followed by single-agent atezolizumab or to receive paclitaxel protein-bound and carboplatin, followed by maintenance pemetrexed at the investigator's discretion (control).[26]
In May 2020, the atezolizumab was approved by the FDA for the first-line treatment of adults with metastatic non-small cell lung cancer (NSCLC) whose tumors have high PD-L1 expression (PD-L1 stained ≥ 50% of tumor cells [TC ≥ 50%] or PD-L1 stained tumor-infiltrating immune cells [IC] covering ≥ 10% of the tumor area [IC ≥ 10%]), with no EGFR or ALK genomic tumor aberrations.[27]
Efficacy was evaluated in IMpower110 (NCT02409342), a multicenter, international, randomized, open-label trial in participants with stage IV NSCLC whose tumors express PD-L1 (TC ≥ 1% or IC ≥ 1%), who had received no prior chemotherapy for metastatic disease.[27] Participants were randomized (1:1) to receive atezolizumab 1200 mg every three weeks until disease progression or unacceptable toxicity or platinum-based chemotherapy.[27]
In May 2020, atezolizumab in combination with bevacizumab was approved by the FDA for people with unresectable or metastatic hepatocellular carcinoma who have not received prior systemic therapy.[28]
Efficacy was investigated in IMbrave150 (NCT03434379), a multicenter, international, open-label, randomized trial in participants with locally advanced unresectable or metastatic hepatocellular carcinoma who had not received prior systemic therapy.[28] A total of 501 participants were randomized (2:1) to receive either atezolizumab 1200 mg as an intravenous infusion (IV) followed by bevacizumab 15 mg/kg IV on the same day, every 3 weeks, or sorafenib orally twice daily.[28]
In July 2020, it was approved in the United States, in combination with cobimetinib and vemurafenib, for the treatment of people with BRAF V600 mutation-positive unresectable or metastatic melanoma.[29]
Efficacy in combination with cobimetinib and vemurafenib was evaluated in a double-blind, randomized (1:1), placebo-controlled, multicenter trial (IMspire150, NCT02908672) in 514 participants.[29] After a 28-day cycle of cobimetinib and vemurafenib, participants received atezolizumab 840 mg intravenous infusion every 2 weeks in combination with cobimetinib 60 mg orally once daily and vemurafenib 720 mg orally twice daily, or placebo in combination with cobimetinib 60 mg orally once daily (21 days on/7 days off) and vemurafenib 960 mg orally twice daily.[29]

IMpower110 randomized patients with stage IV NSCLC with PD-L1 expression ≥ 1% to Atezolizumab single agent or to chemotherapy.[9] The chemotherapy used was Cisplatin or Carboplatin, combined with Gemcitabine for patient with squamous cell NSCLC, or pemetrexed for patients with nonsquamous disease.[9] Atezolizumab was better tolerated than chemotherapy. In the subgroup of patients with EGFR and ALK wild-type tumors who had PD-L1 stained ≥ 50% of tumor cells (205 patients), the overall survival was 20.2 months with Atezolizumab, and 13.1 months with chemotherapy.[9] FDA approval is for patients with PD-L1 stained ≥ 50% of tumor cells, or PD-L1 stained tumor-infiltrating immune cells covering ≥ 10% of the tumor area, with no EGFR or ALK genomic tumor aberrations.[9]
IMpower130 was an open-label, phase 3 trial that compared Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy, with chemotherapy alone as first-line treatment for metastatic non-squamous NSCLC.[9] About half of the patients had PD-L1 negative tumors. Median overall survival was 18.6 months in the atezolizumab plus chemotherapy group and 13.9 months in the chemotherapy group; HR 0.79, p = 0.033.[9] Subgroup analysis showed progression free survival benefit, and a trend toward overall survival benefit in all PD-L1 expression levels.[9]
IMpower150 randomized patients with nonsquamous NSCLC to treatment with chemotherapy plus Bevacizumab, chemotherapy plus atezolizumab or chemotherapy plus Bevacizumab and atezolizumab.[30][9] The chemotherapy used was Carboplatin, and Paclitaxel.[9] Median overall survival was 19.8 and 14.9 months for patients treated with chemotherapy plus Bevacizumab, with or without atezolizumab, respectively.[9] Median OS with Atezolizumab and chemotherapy alone was 19.5 months, raising question with regard to the added value of Bevacizumab to this combination for the general patients population.[9] Importantly, patients with baseline liver metastases had an improved overall survival with Atezolizumab, Bevacizumab, and chemotherapy combination, compared to Bevacizumab and chemotherapy alone, with a median OS of 13.3 and 9.4 months, respectively, HR 0.52.[9] No improvement in overall survival was observed for patients with liver metastasis treated with chemotherapy and atezolizumab compared to patients treated with chemotherapy and Bevacizumab.[9] Recent report about safety and patient-reported outcomes of atezolizumab plus chemotherapy and Bevacizumab shows that this drug combination seems tolerable and with manageable toxicities.[31][9] For patients with nonsquamous NSCLC, with baseline liver metastases, the combination of chemotherapy, Atezolizumab and Bevacizumab could be an important option to consider in the first line.[9]
Society and culture
Drug cost controversy
Atezolizumab treatment costs on average US$13,200 per month in the United States, depending on the dosage schedule.[32] Despite updated data showing 30% more people with extensive stage small cell lung cancer are alive at 24 months compared to those who received chemotherapy alone,[33] Canadian regulator had rejected to fund atezolizumab for extensive stage small-cell lung cancer "as too costly" followed by United Kingdom also citing "drug's cost-effectiveness."[34][35] However, U.K. reversed its previous decision and approved tecentriq for extensive stage small cell lung cancer after price re-think on May 27, 2020.[36][37]
Research
As of 2016, it is in clinical trials for colorectal cancer, melanoma, breast cancer, non-small-cell lung carcinoma, bladder cancer, renal cell carcinoma.[38][39]
Promising results have been observed for melanoma and non-small-cell lung cancer,[40] and bladder cancer.[4]
A phase I trial reported a 19% objective response rate in metastatic triple-negative breast cancer.[41]
As of 2019, atezolizumab is in trial for several types of cancer, such as pancreatic cancer, gastric cancer and ovarian Cancer.[42]
References
- "Tecentriq 1,200 mg concentrate for solution for infusion - Summary of Product Characteristics (SmPC)". (emc). Retrieved 4 March 2020.
- "Tecentriq- atezolizumab injection, solution". DailyMed. 3 June 2020. Retrieved 31 July 2020.
- "Tecentriq EPAR". European Medicines Agency. Retrieved 31 July 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "Genentech Presents Positive Results of Atezolizumab in Advanced Bladder Cancer". 2 October 2015.
- "FDA approves new, targeted treatment for bladder cancer" (Press release). U.S. Food and Drug Administration (FDA). 18 May 2016. Retrieved 20 May 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "EMA reminds physicians to use Tecentriq with nab-paclitaxel for treating breast cancer". European Medicines Agency (EMA). Retrieved 23 May 2021.
- "FDA approves atezolizumab as adjuvant treatment for non-small cell lun". U.S. Food and Drug Administration. 15 October 2021. Retrieved 15 October 2021.
- FDA Professional Drug Information for Tecentriq.
- Nasser, Nicola J.; Gorenberg, Miguel; Agbarya, Abed (November 2020). "First line Immunotherapy for Non-Small Cell Lung Cancer". Pharmaceuticals. 13 (11): 373. doi:10.3390/ph13110373. PMC 7695295. PMID 33171686.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Syn NL, Teng MW, Mok TS, Soo RA (December 2017). "De-novo and acquired resistance to immune checkpoint targeting". The Lancet. Oncology. 18 (12): e731–e741. doi:10.1016/s1470-2045(17)30607-1. PMID 29208439.
- Shields M (11 April 2016). "Roche says FDA fast tracks atezolizumab in specific type of lung cancer". Reuters. Retrieved 11 April 2016.
- "Tecentriq (atezolizumab) Injection". U.S. Food and Drug Administration (FDA). 21 June 2016. Archived from the original on 5 December 2019. Retrieved 4 December 2019.
- "Failed confirmatory trial raises questions about atezolizumab for advanced urothelial cancer". HemOnc Today. 25 June 2017.
- "Checkpoint Inhibitor Use Changed for Bladder Cancer". National Cancer Institute. 26 July 2018.
- "Atezolizumab for Urothelial Carcinoma". U.S. Food and Drug Administration. 18 May 2016. Retrieved 31 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Roche's shocking Tecentriq fail raises red flag for bladder cancer rivals". FiercePharma. 10 May 2017. Retrieved 11 May 2017.
- "Drug Trials Snapshots: Tecentriq". U.S. Food and Drug Administration (FDA). 18 May 2016. Retrieved 31 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves new treatment for non-small cell lung cancer". U.S. Food and Drug Administration (FDA). 18 October 2016. Retrieved 18 May 2016.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - McKee S (8 May 2018). "First-line use of Roche's Tecentriq given priority review". PharmaTimes. Retrieved 8 May 2018.
- "FDA updates prescribing information for Keytruda and Tecentriq". U.S. Food and Drug Administration. 16 August 2018. Retrieved 31 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. (November 2018). "Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer". The New England Journal of Medicine. 379 (22): 2108–2121. doi:10.1056/nejmoa1809615. PMID 30345906.
- "FDA approves atezolizumab with chemotherapy and bevacizumab for first-". U.S. Food and Drug Administration (FDA). 6 December 2018. Retrieved 4 March 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Roche scores first U.S. approval of immunotherapy for breast cancer". Stat. 9 March 2019. Retrieved 9 March 2019.
- "FDA approves atezolizumab for PD-L1 positive unresectable locally advanced or metastatic triple-negative breast cancer". U.S. Food and Drug Administration (FDA) (Press release). 18 March 2019. Archived from the original on 5 December 2019. Retrieved 4 December 2019.
- "FDA approves atezolizumab for extensive-stage small cell lung cancer". U.S. Food and Drug Administration (FDA). 19 March 2019. Archived from the original on 5 December 2019. Retrieved 5 December 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves atezolizumab with nab-paclitaxel and carboplatin for metastatic NSCLC without EGFR/ALK aberrations". U.S. Food and Drug Administration (FDA) (Press release). 3 December 2019. Retrieved 31 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves atezolizumab for first-line treatment of metastatic NSCLC with high PD-L1 expression". U.S. Food and Drug Administration (FDA). 18 May 2020. Retrieved 31 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma". U.S. Food and Drug Administration (FDA). 29 May 2020. Retrieved 31 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "FDA approves atezolizumab for BRAF V600 unresectable or metastatic melanoma". U.S. Food and Drug Administration (Press release). 30 July 2020. Retrieved 31 July 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Socinski, Mark A.; Jotte, Robert M.; Cappuzzo, Federico; Orlandi, Francisco; Stroyakovskiy, Daniil; Nogami, Naoyuki; Rodríguez-Abreu, Delvys; Moro-Sibilot, Denis; Thomas, Christian A.; Barlesi, Fabrice; Finley, Gene (4 June 2018). "Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC". New England Journal of Medicine. 378 (24): 2288–2301. doi:10.1056/NEJMoa1716948. PMID 29863955.
- Reck, Martin; Wehler, Thomas; Orlandi, Francisco; Nogami, Naoyuki; Barone, Carlo; Moro-Sibilot, Denis; Shtivelband, Mikhail; González Larriba, Jose Luis; Rothenstein, Jeffrey; Früh, Martin; Yu, Wei (27 May 2020). "Safety and Patient-Reported Outcomes of Atezolizumab Plus Chemotherapy With or Without Bevacizumab Versus Bevacizumab Plus Chemotherapy in Non–Small-Cell Lung Cancer". Journal of Clinical Oncology. 38 (22): 2530–2542. doi:10.1200/JCO.19.03158. ISSN 0732-183X. PMC 7392741. PMID 32459597.
- "What is the cost of Tecentriq?". Drugs.com.
- "SCLC". Tecentriq. Retrieved 4 March 2020.
- Martell A (20 February 2020). "Exclusive: Canadian regulator considers changes to new drug pricing plan". Reuters. Retrieved 4 March 2020.
- "NICE Cites Cost in Deciding Against Atezolizumab for Frontline Advanced Small Cell Lung Cancer". OncLive. 6 January 2020. Retrieved 4 March 2020.
- "NICE recommends treatment for type of small-cell lung cancer".
- "NICE recommends Roche's Tecentriq in ES-SCLC following price rethink". 27 May 2020.
- "Search of: MPDL3280A". ClinialTrialsGov.
- Bendell JC, Kim TW, Goh BC, Wallin J, Oh DY, Han SW, et al. Clinical activity and safety of cobimetinib (cobi) and atezolizumab in colorectal cancer (CRC). 2016 ASCO Annual Meeting.
- Facchinetti F, Bordi P, Leonetti A, Buti S, Tiseo M (10 September 2018). "Profile of atezolizumab in the treatment of metastatic non-small-cell lung cancer: patient selection and perspectives". Drug Design, Development and Therapy. 12: 2857–2873. doi:10.2147/DDDT.S124380. PMC 6137949. PMID 30237696.
- "MPDL3280A Shows Activity in Triple-Negative Breast Cancer". Cancer Network.
- "Product Development Portfolio". Roche.
External links
- "Atezolizumab". Drug Information Portal. U.S. National Library of Medicine.
- "Atezolizumab". NCI Drug Dictionary. National Cancer Institute.
- "Atezolizumab". National Cancer Institute. 20 May 2016.
- "FDA issues alert about efficacy and potential safety concerns with atezolizumab in combination with paclitaxel for treatment of breast cancer". U.S. Food and Drug Administration (FDA). 8 September 2020.
Clinical trials
- Clinical trial number NCT02541604 for "A Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Immunogenicity, and Preliminary Efficacy of Atezolizumab (Anti-Programmed Death-Ligand 1 [PD-L1] Antibody) in Pediatric and Young Adult Participants With Solid Tumors" at ClinicalTrials.gov
- Clinical trial number NCT02951767 for "A Study of Atezolizumab in Participants With Locally Advanced or Metastatic Urothelial Bladder Cancer (Cohort 1)" at ClinicalTrials.gov
- Clinical trial number NCT02807636 for "Study of Atezolizumab as Monotherapy and in Combination With Platinum-Based Chemotherapy in Participants With Untreated Locally Advanced or Metastatic Urothelial Carcinoma (IMvigor130)" at ClinicalTrials.gov
- Clinical trial number NCT02409342 for "A Study of Atezolizumab (MPDL3280A) Compared With a Platinum Agent (Cisplatin or Carboplatin) + (Pemetrexed or Gemcitabine) in Participants With Stage IV Non-Squamous or Squamous Non-Small Cell Lung Cancer (NSCLC) [IMpower110]" at ClinicalTrials.gov
- Clinical trial number NCT02366143 for "A Study of Atezolizumab in Combination With Carboplatin Plus (+) Paclitaxel With or Without Bevacizumab Compared With Carboplatin+Paclitaxel+Bevacizumab in Participants With Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) (IMpower150)" at ClinicalTrials.gov
- Clinical trial number NCT02367781 for "A Study of Atezolizumab in Combination With Carboplatin Plus (+) Nab-Paclitaxel Compared With Carboplatin+Nab-Paclitaxel in Participants With Stage IV Non-Squamous Non-Small Cell Lung Cancer (NSCLC) (IMpower130)" at ClinicalTrials.gov
- Clinical trial number NCT02008227 for "A Study of Atezolizumab Compared With Docetaxel in Participants With Locally Advanced or Metastatic Non-Small Cell Lung Cancer Who Have Failed Platinum-Containing Therapy (OAK)" at ClinicalTrials.gov
- Clinical trial number NCT02425891 for "A Study of Atezolizumab in Combination With Nab-Paclitaxel Compared With Placebo With Nab-Paclitaxel for Participants With Previously Untreated Metastatic Triple-Negative Breast Cancer (IMpassion130)" at ClinicalTrials.gov
- Clinical trial number NCT03125902 for "A Study of Atezolizumab and Paclitaxel Versus Placebo and Paclitaxel in Participants With Previously Untreated Locally Advanced or Metastatic Triple Negative Breast Cancer (TNBC) (IMpassion131)" at ClinicalTrials.gov
- Clinical trial number NCT02763579 for "A Study of Carboplatin Plus Etoposide With or Without Atezolizumab in Participants With Untreated Extensive-Stage (ES) Small Cell Lung Cancer (SCLC) (IMpower133)" at ClinicalTrials.gov
- Clinical trial number NCT03434379 for "A Study of Atezolizumab in Combination With Bevacizumab Compared With Sorafenib in Patients With Untreated Locally Advanced or Metastatic Hepatocellular Carcinoma [IMbrave150] (IMbrave150)" at ClinicalTrials.gov
- Clinical trial number NCT02908672 for "A Study of Atezolizumab Plus Cobimetinib and Vemurafenib Versus Placebo Plus Cobimetinib and Vemurafenib in Previously Untreated BRAFv600 Mutation-Positive Patients With Metastatic or Unresectable Locally Advanced Melanoma" at ClinicalTrials.gov
- Clinical trial number NCT02486718 for "Study to Assess Safety and Efficacy of Atezolizumab (MPDL3280A) Compared to Best Supportive Care Following Chemotherapy in Patients With Lung Cancer [IMpower010]" at ClinicalTrials.gov