Loncastuximab tesirine
Loncastuximab tesirine , sold under the brand name Zynlonta, is a monoclonal antibody conjugate medication used to treat large B-cell lymphoma.[1] It is an antibody-drug conjugate (ADC) composed of a humanized antibody targeting the protein CD19.[1]
 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Humanized |
| Target | CD19 |
| Clinical data | |
| Pronunciation | /ˌlɒnkæsˈtʌksɪmæb.ˈtɛsɪriːn/ LON-kas-TUK-si-mab TE-si-reen |
| Trade names | Zynlonta |
| Other names | ADCT-402, loncastuximab tesirine-lpyl |
| License data | |
| Routes of administration | Intravenous |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6544H10048N1718O2064S52 |
| Molar mass | 147481.45 g·mol−1 |
Loncastuximab tesirine was approved for medical use in the United States in April 2021.[1][2]
Medical uses
Loncastuximab tesirine is indicated for the treatment of adults with relapsed or refractory large B-cell lymphoma.[1]
Technology
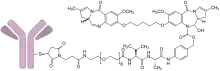
The humanized monoclonal antibody is stochastically conjugated via a valine-alanine cleavable, maleimide linker to a cytotoxic (anticancer) pyrrolobenzodiazepine (PBD) dimer. The antibody binds to CD19, a protein which is highly expressed on the surface of B-cell hematological tumors[3] including certain forms of lymphomas and leukemias. After binding to the tumor cells the antibody is internalized, the cytotoxic drug PBD is released and the cancer cells are killed. PBD dimers are generated out of PBD monomers, a class of natural products produced by various actinomycetes. PBD dimers work by crosslinking specific sites of the DNA, blocking the cancer cells’ division that cause the cells to die. As a class of DNA-crosslinking agents they are significantly more potent than systemic chemotherapeutic drugs.[4]
Clinical trials
Two phase I trials are evaluating the medication in people with relapsed or refractory B-cell non-Hodgkin’s lymphoma and relapsed or refractory B-cell acute lymphoblastic leukemia.[5] At the 14th International Conference on Malignant Lymphoma interim results from a Phase I, open-label, dose-escalating study designed to evaluate the treatment of loncastuximab tesirine in relapsed or refractory non-Hodgkin’s lymphoma were presented.[6] Among the patients enrolled at the time of the data cutoff the overall response rate was 61% in the total patient population (42% complete response and 19% partial response) and in patients with relapsing or refractory diffuse large B-cell lymphoma (DLBCL) the overall response rate was 57% (43% complete response and 14% partial response).[7][8]
History
Loncastuximab tesirine was granted orphan drug designation by the U.S. Food and Drug Administration (FDA) for the treatment of diffuse large B-cell lymphoma.[9]
Society and culture
Legal status
On 15 September 2022, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Zynlonta, intended for the treatment of adults with diffuse large B-cell lymphoma (DLBCL) and high-grade B-cell lymphoma (HGBL).[10] The applicant for this medicinal product is ADC Therapeutics (NL) B.V.[10]
Research
Given its mechanism of action, loncastiximab tesirinine may be appealing in patients ineligible for CAR-T cell therapy.[11]
References
- "Zynlonta- loncastuximab tesirine injection, powder, lyophilized, for solution". DailyMed. Archived from the original on 2 June 2021. Retrieved 1 June 2021.
- "Drug Approval Package: Zynlonta". U.S. Food and Drug Administration (FDA). 24 May 2021. Archived from the original on 2 June 2021. Retrieved 1 June 2021.
- Wang K, Wei G, Liu D (November 2012). "CD19: a biomarker for B cell development, lymphoma diagnosis and therapy". Experimental Hematology & Oncology. 1 (1): 36. doi:10.1186/2162-3619-1-36. PMC 3520838. PMID 23210908.
- "Pyrrolobenzodiazepine". ADC Review. Archived from the original on 2017-08-02. Retrieved 2017-08-02.
- Clinical trial number NCT02669017 for "ADCT-402 in B-NHL" at ClinicalTrials.gov
- Kahl B, Hamadani M, Caimi PF, Reid EG, Havenith K, He S, Feingold JM, O'Connor O (June 2017). "First clinical results of ADCT‐402, a novel pyrrolobenzodiazepine-based antibody drug conjugate (ADC), in relapsed/refractory B‐cell linage NHL" (PDF). Hematol Oncol. 35 (S2): 49–51. doi:10.1002/hon.2437_33.
- "First clinical results of ADCT-402". ADC Review. 16 June 2017. Archived from the original on 2 August 2017. Retrieved 2 August 2017.
- Bainbridge K (3 July 2017). "Grandfather fighting deadly cancer reveals scans of tumors after testing new drug". Mirror. Archived from the original on 28 March 2018. Retrieved 5 April 2018.
- "Loncastuximab tesirine Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 8 June 2017. Archived from the original on 2 June 2021. Retrieved 1 June 2021.
- "Zynlonta : Pending EC decision". European Medicines Agency (EMA). 15 September 2022. Archived from the original on 16 September 2022. Retrieved 18 September 2022. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- Perrone, Salvatore; Lopedote, Paolo; Levis, Mario; Di Rocco, Alice; Smith, Stephen Douglas (2022-02-21). "Management of relapsed or refractory large B-cell lymphoma in patients ineligible for CAR-T cell therapy". Expert Review of Hematology. 15 (3): 215–232. doi:10.1080/17474086.2022.2044778. ISSN 1747-4086. PMID 35184664. S2CID 247010986.
External links
- "Loncastuximab tesirine". Drug Information Portal. U.S. National Library of Medicine.
- "Loncastuximab tesirine-lpyl". NCI Drug Dictionary. National Cancer Institute.
- "Loncastuximab tesirine-lpyl". National Cancer Institute. 21 May 2021.
- Clinical trial number NCT03589469 for "Study to Evaluate the Efficacy and Safety of Loncastuximab Tesirine in Patients With Relapsed or Refractory Diffuse Large B-Cell Lymphoma (LOTIS-2)" at ClinicalTrials.gov