Invasive carcinoma of no special type
Invasive carcinoma of no special type (NST) also known as invasive ductal carcinoma or ductal NOS and previously known as invasive ductal carcinoma, not otherwise specified (NOS) is a group of breast cancers that do not have the "specific differentiating features".[1] Those that have these features belong to other types.[1] While breast cancer is extremely rare in men, invasive carcinoma of no special type is the most commonly diagnosed form of male breast cancer.[2]
| Invasive carcinoma of no special type | |
|---|---|
| Other names | Invasive ductal carcinoma |
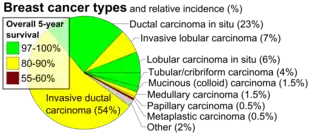 | |
| Histopathologic types of breast cancer, with relative incidences and prognoses, with "invasive ductal carcinoma" at bottom left | |
| Specialty | Oncology, Dermatology, Breast surgery |
In this group are: pleomorphic carcinoma, carcinoma with osteoclast-like stromal giant cells, carcinoma with choriocarcinomatous features, and carcinoma with melanotic features.[1] It is a diagnosis of exclusion, which means that for the diagnosis to be made all the other specific types must be ruled out.[1]
Classification
Invasive carcinoma of no special type (NST) is the most common form of invasive breast cancer. It accounts for 55% of breast cancer incidence upon diagnosis, according to statistics from the United States in 2004.[3] It is also the most common form of breast cancer occurring in men.[2] On a mammogram, it is usually visualized as a mass with fine spikes radiating from the edges. On physical examination, this lump usually feels much harder or firmer than benign breast lesions such as fibroadenoma. On microscopic examination, the cancerous cells invade and replace the surrounding normal tissues. IDC is divided in several histological subtypes.
Signs and symptoms
In many cases, ductal carcinoma is asymptomatic, and detected as abnormal results on mammography. When symptoms occur, a painless, enlarging mass that does not fluctuate with the menstrual period may be felt.[4] : 274–275 Pinching of the overlying skin may also be seen. Certain subtypes, such as inflammatory carcinomas, may result in a swollen, enlarged and tender breast. All variants of cancer, if there is metastatic spread, may cause enlarged lymph nodes and affect other organs.[5] : 746–747
Causes
The cancer may form from the precancerous lesion called ductal carcinoma in situ.[1]
Diagnosis
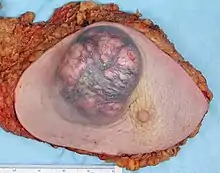

Tumor size
Tumors under 1 cm in diameter are unlikely to spread systemically. Tumors are staged by size.[6]
| Diameter | Tumor size staging number |
|---|---|
| 0–5 mm | T1a |
| 5–10 mm | T1b |
| 10–20 mm | T1c |
| 20-50mm | T2 |
| >50 mm | T3 |
| Tumor involves skin or chest wall | T4 |
Lymph node involvement
Absence of cancer cells in the lymph nodes is a good indication that the cancer has not spread systemically. Presence of cancer in the lymph nodes indicates the cancer may have spread. In studies, some women have had presence of cancer in the lymph nodes, were not treated with chemotherapy, and still did not have a systemic spread. Therefore, lymph node involvement is not a positive predictor of spread.[6]
| Lymph node status | Lymph node involvement grade |
|---|---|
| No involved nodes | N0 |
| Involved node or nodes | N1 |
| Involved nodes that are fixed to one another | N2 |
Clinical staging
Tumor size staging and node involvement staging can be combined into a single clinical staging number.
| Tumor size staging | Node involvement staging | Clinical stage |
|---|---|---|
| T1 | N0 | I |
| T1 | N1 | IIA |
| T2 | N0 | IIA |
| T2 | N1 | IIB |
| T3 | N0 | IIB |
| T1-T2 | N2 | IIIA |
| T3 | N1 | IIIA |
| T3 | N2 | IIIA |
| T4 | N0-N2 | IIIB |
Histopathologic criteria
Carcinomatous cells are seen below the basement membrane of lactiferous ducts. Otherwise, there are no specific histologic characteristics, essentially making it a diagnosis of exclusion.[7]
 Invasive ductal carcinoma of the Breast assayed with anti Mucin 1 antibody.
Invasive ductal carcinoma of the Breast assayed with anti Mucin 1 antibody..jpg.webp) Breast cancer (Infiltrating ductal carcinoma of the breast) assayed with anti HER-2 (ErbB2) antibody.
Breast cancer (Infiltrating ductal carcinoma of the breast) assayed with anti HER-2 (ErbB2) antibody..jpg.webp) Histopathology of invasive ductal carcinoma of the breast representing a scirrhous growth. Core needle biopsy. Hematoxylin and eosin stain.
Histopathology of invasive ductal carcinoma of the breast representing a scirrhous growth. Core needle biopsy. Hematoxylin and eosin stain. Invasive ductal carcinoma of the breast. H&E stain.
Invasive ductal carcinoma of the breast. H&E stain._HER2_expression.JPG.webp) Histopathology of invasive ductal carcinoma of the breast representing a scirrhous growth. Core needle biopsy. HER-2/neu oncoprotein expression by Ventana immunostaining system.
Histopathology of invasive ductal carcinoma of the breast representing a scirrhous growth. Core needle biopsy. HER-2/neu oncoprotein expression by Ventana immunostaining system.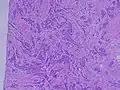 Histopathology of invasive ductal carcinoma of the breast. H&E stain.
Histopathology of invasive ductal carcinoma of the breast. H&E stain.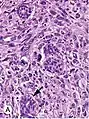 Invasive ductal carcinoma, with occasional entrapped normal ducts (arrow)
Invasive ductal carcinoma, with occasional entrapped normal ducts (arrow)
Grading
The appearance of cancer cells under a microscope is another predictor of systemic spread. The more different the cancer cells look compared to normal duct cells, the greater the risk of systemic spread. There are three characteristics that differentiate cancer cells from normal cells.
- Tendency to form tubular structures
- Nuclear size, shape, and staining intensity
- Mitotic rate - Rate of cell division
The histologic appearance of cancer cells can be scored on these three parameters on a scale from one to three. The sum of these grades is a number between 3 and 9. The score is called a Bloom Richardson Grade (BR) and is expressed [sum of the grades]/9. For example, cells that were graded 2 on all three parameters would result in a BR score of 6/9.
A score of 5 and under is considered Low. 6 to 7 is considered Intermediate. 8 to 9 is considered High.[6]
Vascular invasion
The presence of cancer cell in small blood vessels is called vascular invasion. The presence of vascular invasion increases the probability of systemic spread.[6]
DNA analysis
DNA analysis indicates the amount of DNA in cancer cells and how fast the cancer is growing.
Cells with the normal amount of DNA are called diploid. Cells with too much or too little DNA are called aneuploid. Aneuploid cells are more likely to spread than diploid cells.
DNA testings indicates the rate of growth by determining the number of cells in the synthetic phase (S Phase). An S Phase > 10% means a higher chance of spreading.
The results of DNA testing are considered less reliable predictors of spread than size, histology, and lymph node involvement.[6]
Prognosis
According to the NIH Consensus Conference, if DCIS is allowed to go untreated, the natural course or natural history varies according to the grade of the DCIS. Unless treated, approximately 60 percent of low-grade DCIS lesions will have become invasive at 40 years follow-up.[8] High-grade DCIS lesions that have been inadequately resected and not given radiotherapy have a 50 percent risk of becoming invasive breast cancer within seven years. Approximately half of low-grade DCIS detected at screening will represent overdiagnosis, but overdiagnosis of high-grade DCIS is rare. The natural history of intermediate-grade DCIS is difficult to predict. Approximately one-third of malignant calcification clusters detected at screening mammography already have an invasive focus.
The prognosis of IDC depends, in part, on its histological subtype. Mucinous, papillary, cribriform, and tubular carcinomas have longer survival, and lower recurrence rates. The prognosis of the most common form of IDC, called "IDC Not Otherwise Specified", is intermediate. Finally, some rare forms of breast cancer (e.g., sarcomatoid carcinoma, inflammatory carcinoma) have a poor prognosis. Regardless of the histological subtype, the prognosis of IDC depends also on tumor size, presence of cancer in the lymph nodes, histological grade, presence of cancer in small vessels (vascular invasion), expression of hormone receptors and of oncogenes like HER2/neu.
These parameters can be entered into models that provide a statistical probability of systemic spread. The probability of systemic spread is a key factor in determining whether radiation and chemotherapy are worthwhile. The individual parameters are important also because they can predict how well a cancer will respond to specific chemotherapy agents.
Overall, the five-year survival rate of invasive ductal carcinoma was approximately 85% in 2003.[9]
Treatment
Treatment of invasive carcinoma of no special type (NST) depends on the size of the mass (size of the tumor measured in its longest direction):
- <4 cm mass: surgery to remove the main tumor mass and to sample the lymph nodes in the axilla. The stage of the tumor is ascertained after this first surgery. Adjuvant therapy (i.e., treatment after surgery) may include a combination of chemotherapy, radiotherapy, hormonal therapy (e.g., tamoxifen) and/or targeted therapy (e.g., trastuzumab). More surgery is occasionally needed to complete the removal of the initial tumor or to remove recurrences.
- 4 cm or larger mass: modified (a less aggressive form of radical mastectomy) radical mastectomy (because any malignant mass in excess of 4 cm in size exceeds the criteria for a lumpectomy) along with sampling of the lymph nodes in the axilla.
The treatment options offered to an individual patient are determined by the form, stage and location of the cancer, and also by the age, history of prior disease and general health of the patient. Not all patients are treated the same way.
References
- Sinn HP, Kreipe H (May 2013). "A Brief Overview of the WHO Classification of Breast Tumors, 4th Edition, Focusing on Issues and Updates from the 3rd Edition". Breast Care. 8 (2): 149–154. doi:10.1159/000350774. PMC 3683948. PMID 24415964.
- Zheng G, Leone JP (2022). "Male Breast Cancer: An Updated Review of Epidemiology, Clinicopathology, and Treatment". Journal of Oncology. 2022: 1734049. doi:10.1155/2022/1734049. PMC 9155932. PMID 35656339.
- Percentage values are from United States statistics 2004. Subtype specific incidences are taken from Table 6 (invasive) and Table 3 (in situ) from Eheman CR, Shaw KM, Ryerson AB, Miller JW, Ajani UA, White MC (June 2009). "The changing incidence of in situ and invasive ductal and lobular breast carcinomas: United States, 1999-2004". Cancer Epidemiology, Biomarkers & Prevention. 18 (6): 1763–1769. doi:10.1158/1055-9965.EPI-08-1082. PMID 19454615.. These are divided by total breast cancer incidence (211,300 invasive and 55,700 in situ cases) as reported from Breast Cancer Facts & Figures 2003-2004 "ACS :: Breast Cancer Facts & Figures 2003-2004". Archived from the original on 2009-04-15. Retrieved 2010-06-15.
- Colledge NR, Walker BR, Ralston SH, Britton R, eds. (2010). Davidson's principles and practice of medicine (21st ed.). Edinburgh: Churchill Livingstone/Elsevier. ISBN 978-0-7020-3084-0.
- Robbins basic pathology. Saunders/Elsevier. 2007. ISBN 978-0-8089-2366-4.
- Link J. The Breast Cancer Survival Manual (4th ed.).
- Abdelmessieh P. "Breast Cancer Histology". Medscape. Retrieved 2019-10-04. Updated: May 24, 2018
- Evans A (2004). "Ductal carcinoma in situ (DCIS): are we overdetecting it?". Breast Cancer Research. 6 (Suppl 1): P23. doi:10.1186/bcr842. PMC 3300383.
- NOTE: Article really refers to invasive ductal carcinoma, despite title. Arpino G, Bardou VJ, Clark GM, Elledge RM (2004). "Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome". Breast Cancer Research. 6 (3): R149–R156. doi:10.1186/bcr767. PMC 400666. PMID 15084238.