Oculopharyngeal muscular dystrophy
Oculopharyngeal muscular dystrophy (OPMD) is a rare form of muscular dystrophy with symptoms generally starting when an individual is 40 to 50 years old. It can be autosomal dominant neuromuscular disease or autosomal recessive. The most common inheritance of OPMD is autosomal dominant, which means only one copy of the mutated gene needs to be present in each cell. Children of an affected parent have a 50% chance of inheriting the mutant gene.[2]
| Oculopharyngeal muscular dystrophy | |
|---|---|
| Other names | Muscular dystrophy, oculopharyngeal [1] |
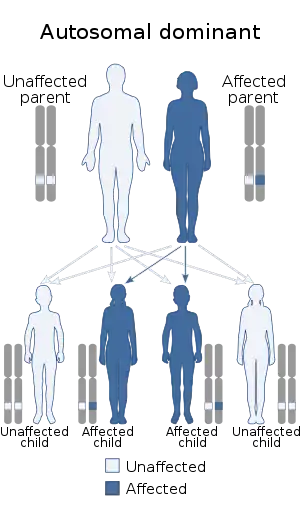 | |
| Autosomal dominant in majority of cases (autosomal recessive minority) | |
| Specialty | Neurology |
| Symptoms | Dysphagia[2] |
| Causes | Mutations on the PABPN1 gene[3] |
| Diagnostic method | Muscle biopsy[2] |
| Treatment | Orthopedic devices for management [1] |
Autosomal dominant inheritance is the most common form of inheritance. Less commonly, OPMD can be inherited in an autosomal recessive pattern, which means that two copies of the mutated gene need to be present in each cell, both parents need to be carriers of the mutated gene, and usually show no signs or symptoms. The PABPN1 mutation contains a GCG trinucleotide repeat [4] at the 5' end of the coding region, and expansion of this repeat which then leads to autosomal dominant oculopharyngeal muscular dystrophy (OPMD) disease.[5][3]
Signs and symptoms
In terms of the signs (and symptoms) of oculopharyngeal muscular dystrophy would be consistent with the following:[2][6]
- Weakness of the extraocular muscles
- Aspiration pneumonia (complication)
- Proximal limb weakness
Though the aforementioned signs/symptoms are the most common, there have been cases though rare, where the peripheral nervous system has had involvement with significant reduction of myelinated fibers [2]
In homozygous cases, this muscular dystrophy is severe and starts earlier in the affected individuals life.[6]
Genetics

The genetics of this type of muscular dystrophy revolve around the PABPN1 gene. This gene suffers mutations that cause the PABPN1 protein to have extra alanine (amino acids), this manifests itself physically in the symptoms of this MD.[3]
The expansion caused by the mutations on the PABPN1 gene ultimately interrupts the cellular mechanics of poly(A) RNA. In most cases oculopharyngeal muscular dystrophy is inherited via autosomal dominance.[7]
The alleles, which are a variant form of a gene[8] involved in this form of MD are: PABPN1, (GCG)n EXPANSION, (GCG)8-13, PABPN1, (GCG)n EXPANSION, (GCG)7 and PABPN1, GLY12ALA.[4]
Diagnosis
The diagnosis of oculopharyngeal muscular dystrophy can be done via two methods, a muscle biopsy or a blood draw with genetic testing for GCG trinucleotide expansions in the PABPN1 gene. The genetic blood testing is more common. Additionally, a distinction between OPMD and myasthenia gravis or mitochondrial myopathy must be made, in regards to the differential diagnosis of this condition.[2]
Treatment
Currently no cure or specific treatment exists to eliminate the symptoms or stop the disease progression. A consistent diet planned with the help of a dietitian along with exercises taught by a speech therapist can assist with mild symptoms of dysphagia. Surgical intervention can also help temporarily manage symptoms related to the ptosis and dysphagia. Cutting one of the throat muscles internally, an operation called cricopharyngeal myotomy, can be one way to ease symptoms in more severe cases. However, for a majority of people, the benefits from such treatments are only temporary. There is currently no treatment available to address the proximal limb weakness. Many of those affected with the proximal limb weakness will eventually require assistive devices such as canes, braces or a wheelchair. As with all surgical procedures, they come with many risk factors.[9][10] As the dysphagia becomes more severe, patients become malnourished, lose significant weight, become dehydrated and suffer from repeated incidents of aspiration pneumonia. These last two are often the cause of death.[11]
Epidemiology
The disease is found across 5 continents (30 countries) and is frequently seen in French Canadians, with a prevalence 1:1000. OPMD affects males and females equally, and affected individuals have been found in Europe (France), Jewish Ashkenazi, and Spanish Americans.[12]
See also
- Muscular dystrophy
- PABPN1
References
- "Oculopharyngeal muscular dystrophy | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Retrieved 3 January 2018.
- Trollet, Capucine; Gidaro, Teresa; Klein, Pierre; Périé, Sophie; Butler-Browne, Gillian; Lacau St Guily, Jean (1993-01-01). Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora J.H.; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C. (eds.). Oculopharyngeal Muscular Dystrophy. Seattle (WA): University of Washington, Seattle. PMID 20301305.update 2014
- Reference, Genetics Home. "oculopharyngeal muscular dystrophy". Genetics Home Reference. Retrieved 2016-05-28.
- "OMIM Entry - * 602279 - POLYADENYLATE-BINDING PROTEIN, NUCLEAR, 1; PABPN1". www.omim.org. Retrieved 2016-05-29.
- https://www.ncbi.nlm.nih.gov/gene/8106 "PABPN1 poly(A) binding protein, nuclear 1 [ Homo sapiens (human) ]"]11 OCT 2014.
- "Oculopharyngeal muscular dystrophy" (PDF). Retrieved 28 May 2016.
- "OMIM Entry - # 164300 - OCULOPHARYNGEAL MUSCULAR DYSTROPHY; OPMD". www.omim.org. Retrieved 2016-05-29.
- Reference, Genetics Home. "What is a gene?". Genetics Home Reference. Retrieved 2016-05-29.
- Davies, Janet E.; Berger, Zdenek; Rubinsztein, David C. (January 2006). "Oculopharyngeal muscular dystrophy: Potential therapies for an aggregate-associated disorder". The International Journal of Biochemistry & Cell Biology. 38 (9): 1457–1462. doi:10.1016/j.biocel.2006.01.016. PMID 16530457.
- Abu-Baker, Aida; Rouleau, Guy A. (February 2007). "Oculopharyngeal muscular dystrophy: Recent advances in the understanding of the molecular pathogenic mechanisms and treatment strategies" (PDF). Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1772 (2): 173–185. doi:10.1016/j.bbadis.2006.10.003. PMID 17110089.
- Malerba, A.; Klein, P.; Bachtarzi, H.; Jarmin, S. A.; Cordova, G.; Ferry, A.; Strings, V.; Espinoza, M. Polay; Mamchaoui, K.; Blumen, S. C.; St Guily, J. Lacau; Mouly, V.; Graham, M.; Butler-Browne, G.; Suhy, D. A.; Trollet, C.; Dickson, G. (31 March 2017). "PABPN1 gene therapy for oculopharyngeal muscular dystrophy". Nature Communications. 8: 14848. Bibcode:2017NatCo...814848M. doi:10.1038/ncomms14848. PMC 5380963. PMID 28361972.
- "Oculopharyngeal muscular dystrophy | Disease | Your Questions Answered | Genetic and Rare Diseases Information Center (GARD) – an NCATS Program". rarediseases.info.nih.gov. Retrieved 2016-05-29.
Further reading
- Raz, Yotam; Raz, Vered (2014-01-01). "Oculopharyngeal muscular dystrophy as a paradigm for muscle aging". Frontiers in Aging Neuroscience. 6: 317. doi:10.3389/fnagi.2014.00317. PMC 4226162. PMID 25426070.
- Hill, M. E.; Creed, G. A.; McMullan, T. F. W.; Tyers, A. G.; Hilton-Jones, D.; Robinson, D. O.; Hammans, S. R. (1 March 2001). "Oculopharyngeal muscular dystrophy". Brain. 124 (3): 522–526. doi:10.1093/brain/124.3.522. ISSN 0006-8950. PMID 11222452.
- Gómez-Torres, Antonio; Abrante Jiménez, Antonio; Rivas Infante, Eloy; Menoyo Bueno, Alicia; Tirado Zamora, Isabel; Esteban Ortega, Francisco (November 2012). "Cricopharyngeal Myotomy in the Treatment of Oculopharyngeal Muscular Dystrophy". Acta Otorrinolaringologica (English Edition). 63 (6): 465–469. doi:10.1016/j.otoeng.2012.11.009. ISSN 2173-5735. PMID 22898142.
- Oculopharyngeal muscular dystrophy at NIH's Office of Rare Diseases