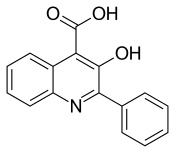
Oxycinchophen
Oxycinchophen is an antirheumatic agent.[1]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.932 |
| Chemical and physical data | |
| Formula | C16H11NO3 |
| Molar mass | 265.268 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
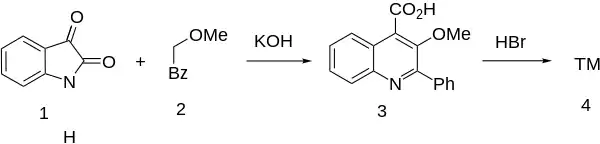
Synthesis
A Pfitzinger-Borsche reaction between isatin (1) and 2-methoxyacetophenone [4079-52-1] (2) catalyzed by potassium hydroxide gives 3-methoxy-2-phenylquinoline-4-carboxylic acid [41957-64-6] (3). Cleavage of the methoxy ether with hydrogen bromide completes the synthesis of oxycinchophen (4).
References
- Iversen M, Munck J, Schourup K (1953). "On the toxicity of 3-hydroxy-2-phenylcinchoninic acid (oxycinchophen); histo-pathological studies in rabbits and guinea-pigs". Acta Pharmacologica et Toxicologica. 9 (3): 215–9. doi:10.1111/j.1600-0773.1953.tb02948.x. PMID 13123940.
- John, Hanns (1932). "Chinolinderivate, XXXVIII. Synthese 2-phenylierter-3-Oxy-chinolin-4-carbonsäuren". Journal für Praktische Chemie. 133 (9-10): 259–272. doi:10.1002/prac.19321330902.
- Frank J. Kreysa, et al. U.S. Patent 2,776,290 (1953 to Chemo Puro Manufacturing Corporation, Long Island, NY.).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.