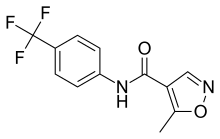
Leflunomide
Leflunomide, sold under the brand name Arava among others, is an immunosuppressive disease-modifying antirheumatic drug (DMARD),[2] used in active moderate-to-severe rheumatoid arthritis and psoriatic arthritis. It is a pyrimidine synthesis inhibitor that works by inhibiting dihydroorotate dehydrogenase.[3]

 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Arava, Lefumide, Arabloc, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a600032 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80%[1] |
| Protein binding | >99%[1] |
| Metabolism | GI mucosa and liver[1] |
| Elimination half-life | 14–18 days[1] |
| Excretion | Faeces (48%), urine (43%)[1] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.123.883 |
| Chemical and physical data | |
| Formula | C12H9F3N2O2 |
| Molar mass | 270.211 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Medical use
Rheumatoid arthritis and psoriatic arthritis are the only indications that have received regulatory approval.[1][4] Arava was developed by Sanofi Aventis and approved by the U.S. Food and Drug Administration in 1998. Clinical studies regarding the following diseases have been conducted:[5] There has been reports on potential re-purposing of leflunomide for treatment of solid tumors with tumor suppressor, PTEN, loss.[6][7] In PTEN negative tumors, leflunomide causes synthetic lethality potentially due to increased demand on pyrimidines in these faster growing cells.[7]
- Polyoma BK virus nephropathy[8]
- Kimura's disease[9]
- Systemic lupus erythematosus[10]
- Felty's syndrome[11]
- Takayasu arteritis[12]
- Granulomatosis with polyangiitis[11]
- Ankylosing spondylitis[13]
- Crohn's disease[14][15]
- Sarcoidosis[16]
- Uveitis[17]
- Still's disease[18]
- Prostate cancer[19]
- Pemphigoid[20]
- Prevention of organ transplant rejection[21]
Side effects
The dose-limiting side effects are liver damage, lung disease and immunosuppression.[21] The most common side effects (occurring in >1% of those treated with it) are, in approximately descending order of frequency:[1][4][22][23][24][25][26] diarrhea, respiratory tract infections, hair loss, high blood pressure, rash, nausea, bronchitis, headache, abdominal pain, abnormal liver function tests, back pain, indigestion, urinary tract infection, dizziness, infection, joint disorder, itchiness, weight loss, loss of appetite, cough, gastroenteritis, pharyngitis, stomatitis, tenosynovitis, vomiting, weakness, allergic reaction, chest pain, dry skin, eczema, paraesthesia, pneumonia, rhinitis, synovitis, cholelithiasis and shortness of breath. Whereas uncommon side effects (occurring in 0.1-1% of those treated with the drug) include:[4] constipation, oral thrush, stomatitis, taste disturbance, thrombocytopenia and hives. Rarely (in 0.1% of those treated with it) it can cause:[4] anaphylaxis, angiooedema, anaemia, agranulocytosis, eosinophilia, leucopenia, pancytopenia, vasculitis, toxic epidermal necrolysis, Stevens–Johnson syndrome, cutaneous lupus erythematosus, severe infection, interstitial lung disease, cirrhosis and liver failure.
Though not reported elsewhere, 80 cases of interstitial pneumonitis involving leflunomide have been reported in Japan between 2003 and 2006. One such case resulting in a death was reported in a 2006 article from Japan and the authors suggest "an inter-racial difference" for the interstitial pneumonitis.[27]
Contraindications
Contraindications include:[1]
- Pregnancy, women of childbearing potential (unless contraception used)
- Liver disease, hepatitis B/C seropositive
- Active serious infections
- Hypersensitivity
Interactions
Other immunomodulatory treatments should be avoided due to the potential for additive immunosuppressant effects, or in the case of immunostimulants like echinacea or astragalus, reduced therapeutic effects.[1] Likewise live vaccines (like haemophilus influenzae type b vaccine and yellow fever vaccines) should be avoided due to the potential for severe infection due to the immunosuppressive nature of the treatment.[1]
The concomitant use of methotrexate, in particular, may lead to severe or even fatal liver-damage or hepatotoxicity. Seventy-five percent of all cases of severe liver damage reported until early 2001 were seen under combined drug therapy leflunomide plus methotrexate.[28] However, some studies have shown that the combination of methotrexate and leflunomide in patients with rheumatoid arthritis gave better results than either drug alone.[28]
Mechanism of action
Leflunomide is an immunomodulatory drug that achieves its effects by inhibiting the mitochondrial enzyme dihydroorotate dehydrogenase (DHODH), which plays a key role in the de novo synthesis of uridine monophosphate (rUMP), which is required for the synthesis of DNA and RNA. Hence, leflunomide inhibits the reproduction of rapidly dividing cells, especially lymphocytes.[21]
The inhibition of human DHODH by teriflunomide, the active metabolite of leflunomide, occurs at levels (approximately 600 nM) that are achieved during treatment of rheumatoid arthritis (RA).[29] Teriflunomide also inhibits several tyrosine kinases.[21] Teriflunomide prevents the expansion of activated and autoimmune lymphocytes by interfering with their cell cycle progression while nonlymphoid cells are able to use another pathway to make their ribonucleotides by use of salvage pyrimidine pathway, which makes them less dependent on de novo synthesis.[29] Teriflunomide also has antiviral effects against numerous viruses including CMV, HSV1 and the BK virus, which it achieves by inhibiting viral replication by interfering with nucleocapsid tegumentation and hence virion assembly.[21]
Pharmacokinetics
It has an oral bioavailability of 80%, protein binding of >99%, metabolism sites of the GI mucosa and liver, volume of distribution (Vd) of 0.13 L/kg, elimination half-life of 14–18 days and excretion routes of faeces (48%) and urine (43%).[1][21][22]
Leflunomide metabolism
Teriflunomide is the main active in vivo metabolite of leflunomide. Upon administration of leflunomide, 70% of the drug administered converts into teriflunomide. The only difference between the molecules is the opening of the isoxazole ring. Upon oral administration of leflunomide in vivo, the isoxazole ring of leflunomide is opened and teriflunomide is formed.[30]
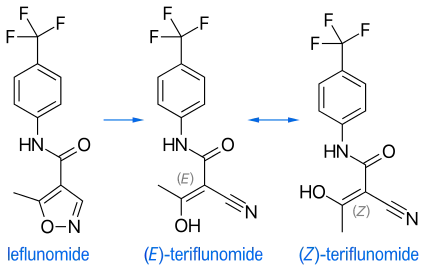
"Regardless of the substance administered (leflunomide or teriflunomide), it is the same molecule (teriflunomide)—the one exerting the pharmacological, immunological or metabolic action in view of restoring, correcting or modifying physiological functions, and does not present, in clinical use, a new chemical entity to patients."[30] Because of this, the European Medicines Agency (EMA) initially had not considered teriflunomide to be a new active substance.[33]
References
- "Arava (leflunomide) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 11 March 2014.
- Dougados M, Emery P, Lemmel EM, Zerbini CA, Brin S, van Riel P (January 2005). "When a DMARD fails, should patients switch to sulfasalazine or add sulfasalazine to continuing leflunomide?". Annals of the Rheumatic Diseases. 64 (1): 44–51. doi:10.1136/ard.2003.016709. PMC 1755199. PMID 15271770.
- Pinto P, Dougados M (2006). "Leflunomide in clinical practice" (PDF). Acta Reumatologica Portuguesa. 31 (3): 215–24. PMID 17094333.
- Rossi S, ed. (2013). Australian Medicines Handbook. Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- "Leflunomide Search". ClinicalTrials.gov. U.S. National Library of Medicine.
- Ozturk S, Mathur D, Zhou RW, Mulholland D, Parsons R (December 2020). "Leflunomide triggers synthetic lethality in PTEN-deficient prostate cancer". Prostate Cancer and Prostatic Diseases. 23 (4): 718–723. doi:10.1038/s41391-020-0251-1. PMC 7666085. PMID 32661432.
- Mathur D, Stratikopoulos E, Ozturk S, Steinbach N, Pegno S, Schoenfeld S, et al. (April 2017). "PTEN Regulates Glutamine Flux to Pyrimidine Synthesis and Sensitivity to Dihydroorotate Dehydrogenase Inhibition". Cancer Discovery. 7 (4): 380–390. doi:10.1158/2159-8290.CD-16-0612. PMC 5562025. PMID 28255082.
- Blanckaert K, De Vriese AS (December 2006). "Current recommendations for diagnosis and management of polyoma BK virus nephropathy in renal transplant recipients". Nephrology, Dialysis, Transplantation. 21 (12): 3364–7. doi:10.1093/ndt/gfl404. PMID 16998219.
- Dai L, Wei XN, Zheng DH, Mo YQ, Pessler F, Zhang BY (June 2011). "Effective treatment of Kimura's disease with leflunomide in combination with glucocorticoids". Clinical Rheumatology. 30 (6): 859–65. doi:10.1007/s10067-011-1689-2. PMID 21286771. S2CID 1914281.
- Wu GC, Xu XD, Huang Q, Wu H (February 2013). "Leflunomide: friend or foe for systemic lupus erythematosus?". Rheumatology International. 33 (2): 273–6. doi:10.1007/s00296-012-2508-z. PMID 22961090. S2CID 7202069.
- Sanders S, Harisdangkul V (April 2002). "Leflunomide for the treatment of rheumatoid arthritis and autoimmunity". The American Journal of the Medical Sciences. 323 (4): 190–3. doi:10.1097/00000441-200204000-00004. PMID 12003373. S2CID 28479334.
- Unizony S, Stone JH, Stone JR (January 2013). "New treatment strategies in large-vessel vasculitis". Current Opinion in Rheumatology. 25 (1): 3–9. doi:10.1097/BOR.0b013e32835b133a. PMID 23114585. S2CID 21101525.
- Haibel H, Rudwaleit M, Braun J, Sieper J (January 2005). "Six months open label trial of leflunomide in active ankylosing spondylitis". Annals of the Rheumatic Diseases. 64 (1): 124–6. doi:10.1136/ard.2003.019174. PMC 1755172. PMID 15608310.
- Prajapati DN, Knox JF, Emmons J, Saeian K, Csuka ME, Binion DG (August 2003). "Leflunomide treatment of Crohn's disease patients intolerant to standard immunomodulator therapy". Journal of Clinical Gastroenterology. 37 (2): 125–8. doi:10.1097/00004836-200308000-00006. PMID 12869881. S2CID 21212960.
- Holtmann MH, Gerts AL, Weinman A, Galle PR, Neurath MF (April 2008). "Treatment of Crohn's disease with leflunomide as second-line immunosuppression : a phase 1 open-label trial on efficacy, tolerability and safety". Digestive Diseases and Sciences. 53 (4): 1025–32. doi:10.1007/s10620-007-9953-7. PMID 17934840. S2CID 29918308.
- Panselinas E, Judson MA (October 2012). "Acute pulmonary exacerbations of sarcoidosis". Chest. 142 (4): 827–836. doi:10.1378/chest.12-1060. PMID 23032450.
- Roy M (August 2007). "Early clinical experience with leflunomide in uveitis". Canadian Journal of Ophthalmology. 42 (4): 634. doi:10.3129/can.j.ophthalmol.i07-085. PMID 17641721.
- Pirildar T (May 2003). "Treatment of adult-onset Still's disease with leflunomide and chloroquine combination in two patients". Clinical Rheumatology. 22 (2): 157. doi:10.1007/s10067-002-0667-0. PMID 12740686. S2CID 41656726.
- Clinical trial number NCT00004071 for "Mitoxantrone and Prednisone With or Without Leflunomide in Treating Patients With Stage IV Prostate Cancer" at ClinicalTrials.gov
- Clinical trial number NCT00802243 for "Leflunomide Associated With Topical Corticosteroids for Bullous Pemphigoid (ARABUL)" at ClinicalTrials.gov
- Teschner S, Burst V (September 2010). "Leflunomide: a drug with a potential beyond rheumatology". Immunotherapy. 2 (5): 637–50. doi:10.2217/imt.10.52. PMID 20874647.
- "PRODUCT INFORMATION ARAVA" (PDF). TGA eBusiness Services. sanofi-aventis australia pty ltd. 7 August 2012. Retrieved 11 March 2014.
- "Arava : EPAR - Product Information" (PDF). European Medicines Agency. Sanofi-Aventis Deutschland GmbH. 21 November 2013. Retrieved 11 March 2014.
- "Data Sheet Arava" (PDF). Medsafe. sanofi-aventis new zealand limited. 29 June 2012. Retrieved 11 March 2014.
- "ARAVA (leflunomide) tablet, film coated [sanofi-aventis U.S. LLC]". DailyMed. sanofi-aventis U.S. LLC. November 2012. Retrieved 11 March 2014.
- "Arava 10mg Tablets - Summary of Product Characteristic". electronic Medicines Compendium. SANOFI. 21 February 2014. Retrieved 11 March 2014.
- Hirabayashi Y, Shimizu H, Kobayashi N, Kudo K (2006). "Leflunomide-induced pneumonitis in a patient with rheumatoid arthritis". Internal Medicine. 45 (10): 689–91. doi:10.2169/internalmedicine.45.1455. PMID 16778342.
- Lee SS, Park YW, Park JJ, Kang YM, Nam EJ, Kim SI, et al. (2009). "Combination treatment with leflunomide and methotrexate for patients with active rheumatoid arthritis". Scandinavian Journal of Rheumatology. 38 (1): 11–4. doi:10.1080/03009740802360632. PMID 19191187. S2CID 205543918.
- Fox RI, Herrmann ML, Frangou CG, Wahl GM, Morris RE, Strand V, Kirschbaum BJ (December 1999). "Mechanism of action for leflunomide in rheumatoid arthritis". Clinical Immunology. 93 (3): 198–208. doi:10.1006/clim.1999.4777. PMID 10600330.
- Melchiorri D, van Zwieten-Boot B, Maciulaitis R, Vilceanu M, Bruins Slot K, Hudson I, Hemmings R, Enzmann H, Demolis P. "Assessment report. AUBAGIO (international non-proprietary name: teriflunomide). Procedure No. EMEA/H/C/002514/0000" (PDF). European Medicines Agency. European Medicines Agency. p. 119. Retrieved 5 June 2015.
- Rozman B (2002). "Clinical pharmacokinetics of leflunomide". Clinical Pharmacokinetics. 41 (6): 421–30. doi:10.2165/00003088-200241060-00003. PMID 12074690. S2CID 33745823.
- "Clinical Pharmacology/Biopharmaceutics Review. Product: ARAVA (leflunomide tablets). Application Number: NDA 20905" (PDF). U.S. Food and Drug Administration. Center for Drug Evaluation and Research. Retrieved 15 April 2016.
- "Summary of Opinion (Initial Authorisation): Aubagio (teriflunomide)" (PDF). European Medicines Agency. Retrieved 15 April 2016.
Further reading
- Shankaranarayana S, Barrett C, Kubler P (February 2013). "The safety of leflunomide". Australian Prescriber. 36 (1): 28–32. doi:10.18773/austprescr.2013.010.