Pegcetacoplan
Pegcetacoplan, sold under the brand name Empaveli among others, is a medication used to treat paroxysmal nocturnal hemoglobinuria (PNH).[2][3][4][5]
 | |
| Clinical data | |
|---|---|
| Trade names | Empaveli, Aspaveli |
| Other names | APL-2 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Subcutaneous |
| Drug class | Complement inhibitor |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
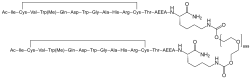
| Formula | C170H248N50O47S4 |
| Molar mass | 3872.40 g·mol−1 |
The most common side effects include injection-site reactions, infections, diarrhea, abdominal pain, respiratory tract infection, viral infection, and fatigue.[2][4]
Paroxysmal nocturnal hemoglobinuria is characterized by red blood cell destruction, anemia (red blood cells unable to carry enough oxygen to tissues), blood clots, and impaired bone marrow function (not making enough blood cells).[3]
Pegcetacoplan is the first treatment for paroxysmal nocturnal hemoglobinuria that binds to and inhibits complement protein C3.[3] Pegcetacoplan was approved for medical use in the United States in May 2021.[3][6] Pegcetacoplan binds to complement protein C3 and its activation fragment C3b with high affinity, thereby regulating the cleavage of C3 and the generation of downstream effectors of complement activation.[4]
Medical uses
Pegcetacoplan is indicated to treat adults with paroxysmal nocturnal hemoglobinuria (PNH).[2][3] Pegcetacoplan is only available for patients who are under the special REMS program.[7] Two weeks before receiving pegcetacoplan, patients must receive vaccinations for pneumonia, meningitis, or influenza type B. Patients who have been vaccinated in the past may still need to get vaccinated two weeks before initiation of the medication.[7]
Pharmacology
Patients with PNH have greater and uninhibited complement activity, which may leads to intravascular (inside blood vessels) or extravascular (within the liver or spleen) hemolysis.[8]
Adverse effects
Meningococcal (a type of bacteria) infections can occur in people taking pegcetacoplan and can become life-threatening or fatal if not treated early.[3] Pegcetacoplan may also predispose individuals to serious infections, especially infections caused by encapsulated bacteria.[3] These infections include but are not limited to Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae.[8] Patients should contact their healthcare team right if way if they experience muscle pain with flu-like symptoms, fever, rash, headache, clammy skin, extreme pain, fast heartbeats, and eye sensitivity to light. Common adverse effects associated with the medication include nausea, diarrhea, cold sores, common-cold like symptoms, tiredness as well as any itching, redness, or sensitivity at the injection site.[7] Pegcetacoplan may cause fetal harm so it should be avoided in pregnant patients.[7] Pegcetacoplan may also interfere with silica reagents in coagulation panels, that can result in patients demonstrating a falsely prolonged activated partial thromboplastin time (aPTT).[8]
History
The effectiveness of pegcetacoplan was evaluated in a study enrolling 80 participants with paroxysmal nocturnal hemoglobinuria and anemia who had been taking eculizumab, a treatment previously approved for paroxysmal nocturnal hemoglobinuria.[3]
Society and culture
Legal status
On 14 October 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Aspaveli, intended for the treatment of adults with paroxysmal nocturnal hemoglobinuria.[9] The applicant for this medicinal product is Swedish Orphan Biovitrum AB (publ).[9] Pegcetacoplan was approved for medical use in the European Union in December 2021.[4]
References
- "Empaveli APMDS". Therapeutic Goods Administration (TGA). 17 February 2022. Retrieved 20 February 2022.
- "Empaveli- pegcetacoplan injection, solution". DailyMed. Archived from the original on 13 July 2021. Retrieved 13 July 2021.
- "FDA approves new treatment for adults with serious rare blood disease". U.S. Food and Drug Administration (FDA). 14 May 2021. Archived from the original on 14 May 2021. Retrieved 14 May 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Aspaveli EPAR". European Medicines Agency (EMA). Archived from the original on 17 December 2021. Retrieved 18 December 2021.
- Hoy SM (August 2021). "Pegcetacoplan: First Approval". Drugs. 81 (12): 1423–1430. doi:10.1007/s40265-021-01560-8. PMID 34342834. S2CID 236884115.
- "Apellis Announces U.S. Food and Drug Administration (FDA) Approval of Empaveli (pegcetacoplan) for Adults with Paroxysmal Nocturnal Hemoglobinuria (PNH)" (Press release). Apellis Pharmaceuticals. 14 May 2021. Archived from the original on 14 May 2021. Retrieved 14 May 2021 – via GlobeNewswire.
- "Pegcetacoplan Uses, Side Effects & Warnings". Drugs.com. Archived from the original on 3 June 2022. Retrieved 3 June 2022.
- "How EMPAVELI works | EMPAVELI® (pegcetacoplan) injection". Empaveli. Archived from the original on 29 November 2021. Retrieved 3 June 2022.
- "Aspaveli: Pending EC decision". European Medicines Agency. 14 October 2021. Archived from the original on 18 October 2021. Retrieved 15 October 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
![]() This article incorporates public domain material from the United States Department of Health and Human Services.
This article incorporates public domain material from the United States Department of Health and Human Services.
External links
- "Pegcetacoplan". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT03500549 for "Study to Evaluate the Efficacy and Safety of APL-2 in Patients With Paroxysmal Nocturnal Hemoglobinuria (PNH)" at ClinicalTrials.gov