Pandemic prevention
Pandemic prevention is the organization and management of preventive measures against pandemics. Those include measures to reduce causes of new infectious diseases and measures to prevent outbreaks and epidemics from becoming pandemics.
It is not to be mistaken for pandemic preparedness or mitigation (e.g. against COVID-19) which largely seek to mitigate the magnitude of negative effects of pandemics, although the topics may overlap with pandemic prevention in some respects.
Some efforts of pandemic prevention reportedly risk triggering pandemics while not engaging in any form of pandemic prevention is risky as well.
History
2002–2004 SARS outbreak
During the 2002–2004 SARS outbreak, the SARS-CoV-1 virus was prevented from causing a pandemic of Severe acute respiratory syndrome (SARS). Rapid action by national and international health authorities such as the World Health Organization helped to slow transmission and eventually broke the chain of transmission, which ended the localized epidemics before they could become a pandemic. Human-to-human transmission of SARS may be considered eradicated, however, it could re-emerge as SARS-CoV-1 probably persists as a potential zoonotic threat in its original animal reservoir.[1] This warrants monitoring and reporting of suspicious cases of atypical pneumonia.[2] Effective isolation of patients was enough to control spread because infected individuals usually do not transmit the virus until several days after symptoms begin and are most infectious only after developing severe symptoms.[3]
MERS-CoV/NeoCoV alert
In January 2022, Chinese scientists at the Wuhan University and other institutions reported in a preprint the detection of the closest MERS-CoV relative in bats to date, NeoCoV, and PDF-2180-CoV that can efficiently use bats' ACE2 for cell-entry. The to-date unreviewed preprint finds that one mutation could result in a 'MERS-CoV-2' that, like SARS-CoV-2, can use humans' ACE2 receptor and at least initially has both a high fatality (MERS-CoV had a mortality of around 35%)[4] and high transmission rate, and hence represents a risk to biosafety and of potential zoonotic spillover.[5][6] According to one report, the WHO stated that further study would be required to find out "whether the virus detected in the study will pose a risk for humans".[7] The study also emphasizes the need for pathogen/spillover surveillance.[8][6]
Other
- Monkeypox: On 21 May 2022, the WHO informed about the international 2022 monkeypox outbreak in non-endemic countries[9] – an unprecedented number of cases detected outside of Africa[10] after the first of these cases was detected on 6 May.[11] On 24 May, the WHO stated that the outbreak can be contained.[12] The main method used for the early containment is 'ring vaccination' – vaccinating close contacts of positive cases via already-existing vaccines alongside pre-exposure vaccination of people at higher risk.[10][13][14]
Measures
Infrastructure and international development
Robust, collaborating public health systems that have the capacity for active surveillance for early detection of cases and to mobilize their health care coordination capacity may be required to be able stop contagion promptly.[15][16][17] After an outbreak there is a certain window of time during which a pandemic can still be stopped by the competent authorities isolating the first infected and/or fighting the pathogen. A good global infrastructure, consequent information exchange, minimal delays due to bureaucracy and effective, targeted treatment measures can be prepared.[18] In 2012 it has been proposed to consider pandemic prevention as an aspect of international development in terms of health-care infrastructure and changes to the pathogen-related dynamics between humans and their environment including animals.[19] Often local authority carers or doctors in Africa, Asia or Latin America register uncommon accumulations (or clusterings) of symptoms but lack options for more detailed investigations.[20] Scientists state that "research relevant to countries with weaker surveillance, lab facilities and health systems should be prioritized" and that "in those regions, vaccine supply routes should not rely on refrigeration, and diagnostics should be available at the point of care".[21] Two researchers have suggested that public health systems "in each country" need to be capable of detecting contagion early, diagnosing it accurately, implementing effective disease control measures, and fully collaborating with the relevant international authorities at each stage .[22] U.S. officials have proposed a range of reforms to international health regulations and global institutions for global health security.[23]
Technology-centric measures
Biosafety technologies and biotechnology regulation
Potential policies that support global biosafety could make use of various technologies, including but not limited to laboratory containment technologies. Proposals to increase biosafety in terms of laboratories, scientific field work and research and development-related activities include:
- limiting research on highly contagious biological agents to only trained researchers in well-protected environments and advanced biological safety systems and disposal of biohazards.[24]
- improving physical security and educating scientists about the misuse potentials[25]
- review processes that ensure risks are justified and minimized, such as preventing certain gain-of-function studies[26][27] (the exact definition of "gain-of-function" is contested and there also the term "enhanced potential pandemic pathogens").[28] Arguments for gain-of-function-type research may include "that vaccines and therapeutics can be pre-emptively researched and developed" this way.[29]
- monitoring and strengthening laboratory protocols around the world[30]
- Work on coronaviruses at the Wuhan Institute of Virology was carried out at biosafety level 2 (with level 4 being the most secure)[31]
- According to a study "there are no national guidelines or reference standards available in India on certification and validation of biosafety laboratories"[32]
- According to an expert, there "really isn't an agreed to and followed set of standards for how [biosafety level] BSL3 and BSL4 labs should secure themselves"[29]
- In a 2018 study it was suggested that there is a need "to update international laboratory biosafety guidance" "to globalize biosafety"[33]
- monitoring and strengthening field work protocols around the world (such as viral sampling)[34]
- The so far closest known relative virus (with a 96.8% similarity) to SARS-CoV-2 was found in samples from wild horseshoe bats in/at caves in northern Laos.[35][36] No SARS-CoV-2 related viruses could be found in any samples collected in China, including from the only two domestic caves where RaTG13 and RmYN02 were detected, indicating such viruses may currently not circulate in bats in the country.[35][37]
- A small survey reported that many biosafety professionals conducting field collection of potentially infectious specimens have not been formally trained on the topic[38]
- There are multiple known examples of field-associated infections[39]
- making deadly viruses harder to engineer[40]
- global efforts to end research into developing dangerous new diseases[40]
- measures that don't rely on relevant technological equipment and biotechnology products (as well as data and knowledge) only being available to registered scientists and all of these scientists to act responsibly may also be possible
- According to one expert, the "international bioweapons community" should work towards having supply chain choke points identified and help implement robust monitoring of them, such as, for example, key input material[29]
- One international team plans to make DNA synthesis screening available for free to countries worldwide and could establish a level of safety if regulations require that DNA synthesis companies send sequences for screening against a certified database[40]
- Some companies that manufacture DNA started collaboration to limit access to dangerous genes so that only authorized laboratories can obtain DNA of "about 60" lethal germs and toxins[41]
Risks of pandemic prevention
Efforts of pandemic prevention and related endeavors reportedly risk triggering pandemics themselves as of 2021. These risk include, but are not limited to, unwitting laboratory escape and accidents such as spillovers during field interventions/experiments like field collection,[34][24] and misuse of its results due to e.g. insecure commercial sales of required equipment and/or materials and/or data.
One approach to mitigate risks from pandemic prevention is to "maintain a database with hashes of deadly and dangerous sequences" which don't contain data with a potential for danger (depending on various factors) and also "can't be reverse-engineered to learn the dangerous original sequence if you don't already know it". This would theoretically enable checking sequences against a database of recorded pathogens without maintaining a database of deadly sequences.[40]
Pathogen detection and prediction
.png.webp)
In a 2012 study it is claimed that "new mathematical modelling, diagnostic, communications, and informatics technologies can identify and report hitherto unknown microbes in other species, and thus new risk assessment approaches are needed to identify microbes most likely to cause human disease". The study investigates challenges in moving the global pandemic strategy from response to pre-emption.[43] Some scientists are screening blood samples from wildlife for new viruses.[44] The international Global Virome Project (GVP) aims to identify the causes of fatal new diseases before emergence in human hosts by genetically characterizing viruses found in wild animals.[45] The project aims to enlist an international network of scientists to collect hundreds of thousands of viruses, map their genomes, characterize and risk-stratify them to identify which ones to pay attention to. However, some infectious disease experts have criticized the project as too broad and expensive due to limited global scientific and financial resources and because only a small percentage of the world's zoonotic viruses may cross into humans and pose a threat. They argue for prioritizing rapidly detecting diseases when they cross into humans and an improving the understanding of their mechanisms.[46] A successful prevention of a pandemic from specific viruses may also require ensuring that it does not re-emerge – for instance by sustaining itself in domestic animals.[47]
Pathogen detection mechanisms may allow the construction of an early warning system which could make use of artificial intelligence surveillance and outbreak investigation.[17] Edward Rubin notes that after sufficient data has been gathered artificial intelligence could be used to identify common features and develop countermeasures and vaccines against whole categories of viruses.[45] It might be possible to predict viral evolution using machine learning.[48] In April 2020 it was reported that researchers developed a predictive algorithm which can show in visualizations how combinations of genetic mutations can make proteins highly effective or ineffective in organisms – including for viral evolution for viruses like SARS-CoV-2.[49][50] In 2021, pathogen researchers reported the development of machine learning models for genome-based early detection and prioritization of high-risk potential zoonotic viruses in animals prior to spillover to humans which could be used for virus surveillance for (i.a.) measures of "early investigation and outbreak preparedness" and, according to the study, would have been capable of predicting SARS-CoV-2 as a high-risk strain without prior knowledge of zoonotic SARS-related coronaviruses.[51][52]
An artificial "global immune system"-like technological system that includes pathogen detection may be able to substantially reduce the time required to take on a biothreat agent.[53] A system of that sort would also include a network of well-trained epidemiologists who could be rapidly deployed to investigate and contain an outbreak.[17]
Funding for the United States' PREDICT government research program that sought to identify animal pathogens that might infect humans and to prevent new pandemics was cut in 2019.[54] Funding for United States' CDC programs that trained workers in outbreak detection and strengthened laboratory and emergency response systems in countries where disease risks are greatest to stop outbreaks at the source was cut by 80% in 2018.[55]
In 2022, researchers reported the development of an ultra-high-throughput sequence alignment technology that enables searching the planetary collection of nucleic acid sequences. The open source supercomputing-based Serratus Project identified over 130,000 RNA-based viruses, including 9 coronaviruses. While such and related endeavors and data are reportedly risky themselves as of 2021,[34][24] the project aims to improve pathogen surveillance, the understanding of viral evolutionary origins and enable quickly connecting strange emerging illnesses to recorded viruses.[56][57]
Despite recent advances in pandemic modeling, experts using mostly experience and intuition are still more accurate in predicting the spread of disease than strictly mathematical models.[58]
CRISPR-based immune subsystems
In March 2020 scientists of Stanford University presented a CRISPR-based system, called PAC-MAN (Prophylactic Antiviral Crispr in huMAN cells), that can find and destroy viruses in vitro. However, they weren't able to test PAC-MAN on the actual SARS-CoV-2, use a targeting-mechanism that uses only a very limited RNA-region, haven't developed a system to deliver it into human cells and would need a lot of time until another version of it or a potential successor system might pass clinical trials. In the study published as a preprint they write that it could be used prophylactically as well as therapeutically. The CRISPR-Cas13d-based system could be agnostic to which virus it's fighting so novel viruses would only require a small change.[59][60] In an editorial published in February 2020 another group of scientists claimed that they have implemented a flexible and efficient approach for targeting RNA with CRISPR-Cas13d which they have put under review and propose that the system can be used to also target SARS-CoV-2 in specific.[61] There have also been earlier successful efforts in fighting viruses with CRISPR-based technology in human cells.[62][63] In March 2020 researchers reported that they have developed a new kind of CRISPR-Cas13d screening platform for effective guide RNA design to target RNA. They used their model to predict optimized Cas13 guide RNAs for all protein-coding RNA-transcripts of the human genome's DNA. Their technology could be used in molecular biology and in medical applications such as for better targeting of virus RNA or human RNA. Targeting human RNA after it's been transcribed from DNA, rather than DNA, would allow for more temporary effects than permanent changes to human genomes. The technology is made available to researchers through an interactive website and free and open source software and is accompanied by a guide on how to create guide RNAs to target the SARS-CoV-2 RNA genome.[64][65]
Scientists report to be able to identify the genomic pathogen signature of all 29 different SARS-CoV-2 RNA sequences available to them using machine learning and a dataset of 5000 unique viral genomic sequences. They suggest that their approach can be used as a reliable real-time option for taxonomic classification of novel pathogens.[66][67]
Testing and containment

Timely use and development of quick testing systems for novel virus in combination with other measures might (possibly) make it possible to end transmission lines of outbreaks before they become pandemics.[68][69][70][71] After an outbreak there may be a certain window of time during which a pandemic can still be prevented.[18] A key difficulty with early detection and containment is that in the globalized and urbanized world, pathogens can spread rapidly to several regions worldwide via travel,[72][73] before it may be possible to notice them and e.g. initiate contact-tracing and containment measures. Rapid communication of data for health systems to implement any public intervention measures may be important.[74] A "'One Health' global network for proactive surveillance, rapid detection, and prevention of MERS-CoV and other epidemic infectious diseases threats" has been proposed in 2016.[75]
Moreover, there are several issues with tests. For example, a high discovery-rate is important. For instance, this is the reason why no thermal scanners with a low discovery-rate were used in airports for containment during the 2009 swine flu pandemic.[76] Coverage may also be important. (See also: wastewater-based epidemiology and pooled COVID-19 populations testing, possibly based on CRISPR[24])
Some argue that the best forms of prevention for natural nonsynthetic viruses would be stopping the viruses from spilling into humans in the first place, rather than trying to contain outbreaks.[77]
The German program InfectControl 2020 seeks to develop strategies for prevention, early recognition and control of infectious diseases.[78][79] In one of its projects "HyFly" partners of industry and research work on strategies to contain chains of transmission in air traffic, to establish preventive countermeasures and to create concrete recommendations for actions of airport operators and airline companies. One approach of the project is to detect infections without molecular-biological methods during passenger screening. For this researchers of the Fraunhofer-Institut for cell therapy and immunology are developing a non-invasive procedure based on ion-mobility spectrometry (IMS).[80]
Incentives for countries to report new viruses may be important for sufficiently fast detection and for avoiding cover-ups. A global treaty proposed by the E.U. could address this issue.[81] Rapid regional, possibly also national, capacities in terms of e.g. means, mobile laboraties or diagnostics,[82][83] personnel, technologies, financial insurances and coordination[84] may also be important.
In cases where vaccines already exist a major method for early containment is 'ring vaccination' – vaccinating close contacts of positive cases (and/or geographical areas) via existing vaccines as well as pre-exposure vaccination of people at higher risk.[10][13][14] There are also precautionary vaccine stockpiles.[85] Production capacities may also be important.[86] See also: vaccine-to-variant adjustment for SARS-CoV-2 Omicron
Researchers have developed a portable virus capture device, coupled with label-free Raman spectroscopy for identification of newly emerging or circulating viruses as a major first step toward managing the public health response to potential outbreaks. It could rapidly obtain the Raman signature of a virus and use machine learning to recognize the virus based on its weighted combination Raman spectrum fingerprint, being able to distinguish between influenza virus type A versus type B.[71]
Surveillance and mapping
Viral hotspots and zoonotic genomics
Monitoring people who are exposed to animals in viral hotspots – including via virus monitoring stations – can register viruses at the moment they enter human populations - this might enable prevention of pandemics.[87] The most important transmission pathways often vary per underlying driver of emerging infectious diseases such as the vector-borne pathway and direct animal contact for land-use change – the leading driver for emerging zoonoses by number of emergence events as defined by Jones et al. (2008).[88] 75% of the reviewed 1415 species of infectious organisms known to be pathogenic to humans account for zoonoses by 2001.[89][90] Genomics could be used to precisely monitor virus evolution and transmission in real time across large, diverse populations by combining pathogen genomics with data about host genetics and about the unique transcriptional signature of infection.[91] The "Surveillance, Outbreak Response Management and Analysis System" (SORMAS) of the German Helmholtz-Zentrum für Infektionsforschung (HZI) and Deutsches Zentrum für Infektionsforschung (DZIF), who collaborate with Nigerian researchers, gathers and analyzes data during an outbreak, detects potential threats and allows to initiate protective measures early. It's meant specifically for poorer regions and has been used for the fight against a monkeypox outbreak in Nigeria.[92][93]
Improving "frontline healthcare provision and testing capacity for deprived communities around the world" could enable detecting, identifying and controlling outbreaks without delays .[94]
Syndromic surveillance and border control
Expert on infectious diseases at the Johns Hopkins Center for Health Security, Amesh Adalja states that the most immediate way to predict a pandemic is with deeper surveillance of symptoms that fit the virus' profile.[46] The scientific and technological ways of quickly detecting a spillover could be improved so that an outbreak can be isolated before it becomes an epidemic or pandemic.[95] David Quammen states that he heard about the idea to develop technology to screen people at airport security points for whether or not they carry an infectious disease ten years ago and thought it was going to be done by now.[95] Thermometers whose measurement data is directly shared via the Internet and medical guidance apps have been used to plot and map unusual fever levels to detect anomalous outbreaks.[96] Various forms of data-sharing could be added to health care institutions such as hospitals so that e.g. anonymized data about symptoms and incidences found to be unusual or characteristic of a pandemic threat could enable high-resolution "syndromic surveillance" as an early warning system. In 1947, the World Health Organization established such a global network of some hospitals.[97][98] Such sharing and off-site evaluation of symptoms and possibly related medical data may have complementary benefits such as improving livelihoods of workers who work with livestock[99] and improving the accuracy, timeliness and costs of disease prognoses. The WHO Hub for Pandemic and Epidemic Intelligence is an early-warning center that attempts to aggreggate data and quickly analyze it to predict, prevent, detect, prepare for, and respond to outbreaks and was set up Berlin in September 2021. It uses machine learning and may analyze data about animal health, unusual symptoms in humans, migration and other related developments that may contain detectable patterns.[100][101]
Mutation surveillance
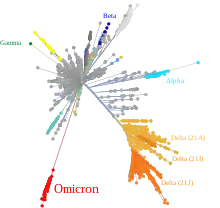
In December 2020 during the COVID-19 pandemic, national and international officials reported mutated variants of SARS-CoV-2, including some with higher transmissibility and worldwide spread. While mutations are common for viruses and the spread of some of the virus' mutations have been tracked earlier, mutations that make it more transmittable or severe can be problematic. Resources for disease surveillance have improved during the pandemic so that medical systems around the world are starting to be equipped to detect such mutations with genomic surveillance in a manner relevant to pandemic mitigation and the prevention of sub-pandemics of specific variants or types of variants. As of December 2020, contemporary measures such as COVID-19 vaccines and medications seem to be effective in the treatment of infections with the tracked mutated variants compared to earlier forms that are closer to the original virus/es.[102][103][104][105] Tools used in the pandemized outbreak of COVID-19 included PANGOLIN[106] and Nextstrain.[107] In July 2021, scientists reported the detection of anomalous unnamed unknown-host SARS-CoV-2 lineages via wastewater surveillance.[108][109]
Genomic surveillance refers to monitoring pathogens and analyzing their genetic similarities and differences, which may enable (early) alerts and tailoring interventions, countermeasures and recommendations for the public, like vaccines.[110][111] In terms of pandemic prevention, it may be especially useful for vaccine-preventable diseases.[111] A problem with the surveillance for mutated variants during the COVID-19 pandemic was that entities don't have sufficient incentives (and/or requirements) to report such variants. A global treaty proposed by the E.U. includes such incentives.[81] A further issue was that vaccines did not provide a high enduring protection against the variants. One approach to solve this problem are pan-virus vaccines that protect against many strains (in this case a pan-SARS-CoV-2-like/variant-coronavirus vaccine), possibly including variants that do not yet exist.[112]
Policy and economics
A 2014 analysis asserts that "the window of opportunity to deal with pandemics as a global community is within the next 27 years. Pandemic prevention therefore should be a critical health policy issue for the current generation of scientists and policymakers to address.[113] A 2007 study warns that "the presence of a large reservoir of SARS-CoV-like viruses in horseshoe bats, together with the culture of eating exotic mammals in southern China, is a time bomb. The possibility of the reemergence of SARS and other novel viruses from animals or laboratories and therefore the need for preparedness should not be ignored".[114][89] The US' National Security Council Directorate for Global Health Security and Biodefense, which worked on preparing for the next disease outbreak and preventing it from becoming an epidemic or pandemic, was closed in 2018.[115][116] A study concluded that the three practical actions "better surveillance of pathogen spillover and development of global databases of virus genomics and serology, better management of wildlife trade, and substantial reduction of deforestation" would have a highly favorable cost-benefit ratio.[117] A second study affirms that if policy priorities were refocused from disease control to prevention, implementing such proactive actions would "cost a very small fraction of the reconstruction budgets".[73]
Environmental policy and economics
Some experts link pandemic prevention with environmental policy and caution that environmental destruction as well as climate change drives wildlife to live close to people.[89][118]
Climate change
The WHO projects that climate change will also affect infectious disease occurrence.[119] It is projected that interspecies viral sharing, that can lead to novel viral spillovers, will increase due to ongoing climate change-caused geographic range-shifts of mammals (most importantly bats). Risk hotspots would mainly be located at "high elevations, in biodiversity hotspots, and in areas of high human population density in Asia and Africa".[120] A 2016 study reviews literature on the evidences for the impact of climate change on human infectious disease, suggests a number of proactive measures for controlling health impacts of climate change and finds that climate change impacts human infectious disease via alterations to pathogen, host and transmission.[121]
Another way climate change may affect pandemic risks, is by pathogens in thawing permafrost (e.g. in the Arctic) that may have infected now-extinct ancestral humans in such regions.[122] However, a scientist concluded that probably permafrost per se shouldn't host more pathogens than any other environment.[123] Nevertheless, the risk from permafrost pathogens is unknown and viruses from the very first humans to populate the Arctic could emerge.[124] Moreover, researchers have suggested more work on microbes soon to be released from melting glaciers across the world to identify and understand potential threats in advance.[125][126]
Ecosystem degradation and consumption

Studies have shown that the risk of disease outbreaks can increase substantially after forests are cleared.[127][128][129][130] The likelihood of human-nonhuman primates contact events is increased jointly by forest landscape fragmentation and certain smallholders' behaviors in forest patches.[131] Loss of biodiversity may remove natural regulation of viruses and make fleeing animals meet other species for the first time.[94] According to Kate Jones, chair of ecology and biodiversity at University College London, the disruption of pristine forests driven by logging, mining, road building through remote places, rapid urbanisation and population growth is bringing people into closer contact with animal species they may never have been near before, resulting in transmission of diseases from wildlife to humans.[132] An August 2020 study published in Nature concludes that the anthropogenic destruction of ecosystems for the purpose of expanding agriculture and human settlements reduces biodiversity and allows for smaller animals such as bats and rats, who are more adaptable to human pressures and also carry the most zoonotic diseases, to proliferate. This in turn can result in more pandemics.[133] In October 2020, the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services published its report on the 'era of pandemics' by 22 experts in a variety of fields, and concluded that anthropogenic destruction of biodiversity is paving the way to the pandemic era, and could result in as many as 850,000 viruses being transmitted from animals – in particular birds and mammals – to humans. The increased pressure on ecosystems is being driven by the "exponential rise" in consumption and trade of commodities such as meat, palm oil, and metals, largely facilitated by developed nations, and by a growing human population. According to Peter Daszak, the chair of the group who produced the report, "there is no great mystery about the cause of the Covid-19 pandemic, or of any modern pandemic. The same human activities that drive climate change and biodiversity loss also drive pandemic risk through their impacts on our environment."[134]
Stanford biological anthropologist James Holland Jones notes that humanity has "engineer[ed] a world where emerging infectious diseases are both more likely and more likely to be consequential", referring to the modern world's prevalent highly mobile lifestyles, increasingly dense cities, various kinds of human interactions with wildlife and alterations of the natural world.[135] Furthermore, when multiple species that are not usually next to each other are driven to live closely together new diseases may emerge.[47] Research shows that abundant animals, plants, insects, and microbes living in complex, mature ecosystems can limit the spread of disease from animals to people.[136] The United Nations is formulating nature-focused action plans that could help to stop the next pandemic before it starts. These strategies include conserving ecosystems and wilderness that are still untouched by human activity, and restoring and protecting significant areas of land and ocean (i.e. through protected areas).[137] Protected areas (which may hold wildlife) also limits human presence and/or limits the exploitation of resources (including non-timber forest products such as game animals, fur-bearers, ...).[138] An article by the World Economic Forum states that studies have shown that deforestation and loss of wildlife cause increases in infectious diseases and concludes that the recovery from the COVID-19 pandemic should be linked to nature recovery, which it considers economically beneficial.[139]
Dennis Caroll of the Global Virome Project stated that the "extractive industry — oil and gas and minerals, and the expansion of agriculture, especially cattle" are the biggest predictors of where spillovers can be seen.[46] A study proposes that policy responses "addressing zoonotic threats should include ecosystem regeneration".[140]
In the 2000s, a WHO spokesperson summarized the animal-related aspects of pandemics, stating "the whole relationship between the animal kingdom and the human kingdom is coming under stress".[141]
The integrated, unifying approach of One Health adresses at health of people, animals and the environment at once. It could "boost risk identification, reduction, and surveillance in animals and at the human-animal-environment interface".[30]
Data on current causes of emerging diseases
.jpg.webp)
A study which was published in April 2020 and is part of the PREDICT program found that "virus transmission risk has been highest from animal species that have increased in abundance and even expanded their range by adapting to human-dominated landscapes", identifying domesticated species, primates and bats as having more zoonotic viruses than other species and "provide further evidence that exploitation, as well as anthropogenic activities that have caused losses in wildlife habitat quality, have increased opportunities for animal–human interactions and facilitated zoonotic disease transmission".[142]
An UN Environment report presents the causes of the emerging diseases with a large share being environmental:[143]
| Cause | Part of emerging diseases caused by it (%) |
|---|---|
| Land-use change | 31% |
| Agricultural industry changes | 15% |
| International travel and commerce | 13% |
| Medical industry changes | 11% |
| War and Famine | 7% |
| Climate and Weather | 6% |
| Human demography and behavior | 4% |
| Breakdown of public health | 3% |
| Bushmeat | 3% |
| Food industry change | 2% |
| Other | 4% |
The report also lists some of the latest emerging diseases and their environmental causes:[143]
| Disease | Environmental cause |
|---|---|
| Rabies | Forest activities in South America |
| Bat associated viruses | Deforestation and Agricultural expansion |
| Lyme disease | Forest fragmentation in North America |
| Nipah virus infection | Pig farming and intensification of fruit production in Malaysia |
| Japanese encephalitis virus | irrigated rice production and pig farming in Southeast Asia |
| Ebola virus disease | Forest losses |
| Avian influenza | Intensive Poultry farming |
| SARS virus | contact with civet cats either in the wild or in live animal markets |
According to a 2001 study and its criteria a total of 1415 species of infectious agents in 472 different genera have been reported to date to cause disease in humans. Out of these reviewed emerging pathogen species 75% are zoonotic. A total of 175 species of infectious agents from 96 different genera are associated with emerging diseases according its criteria. Some of these pathogens can be transmitted by more than one route. Data on 19 categories of the 26 categories which contained more than 10 species includes:[90]
| Transmission route | Zoonotic status | Taxonomic division | Total number of species | Number of emerging species | Proportion of species emerging |
|---|---|---|---|---|---|
| indirect contact | zoonotic | viruses | 37 | 17 | 0.459 |
| indirect contact | zoonotic | protozoa | 14 | 6 | 0.429 |
| direct contact | zoonotic | viruses | 63 | 26 | 0.413 |
| direct contact | non-zoonotic | protozoa | 15 | 6 | 0.400 |
| indirect contact | non-zoonotic | viruses | 13 | 4 | 0.308 |
| direct contact | non-zoonotic | viruses | 47 | 14 | 0.298 |
| vector borne | zoonotic | viruses | 99 | 29 | 0.293 |
| vector borne | zoonotic | bacteria | 40 | 9 | 0.225 |
| indirect contact | zoonotic | bacteria | 143 | 31 | 0.217 |
| vector borne | zoonotic | protozoa | 26 | 5 | 0.192 |
| direct contact | zoonotic | bacteria | 130 | 20 | 0.154 |
| indirect contact | zoonotic | fungi | 85 | 11 | 0.129 |
| direct contact | zoonotic | fungi | 105 | 13 | 0.124 |
| vector borne | zoonotic | helminths | 23 | 2 | 0.087 |
| direct contact | non-zoonotic | bacteria | 125 | 7 | 0.056 |
| indirect contact | non-zoonotic | bacteria | 63 | 3 | 0.048 |
| indirect contact | non-zoonotic | fungi | 120 | 3 | 0.025 |
| direct contact | non-zoonotic | fungi | 123 | 3 | 0.024 |
| indirect contact | zoonotic | helminths | 250 | 6 | 0.024 |
Bioresearch and development regulation

Toby Ord, author of the book The Precipice: Existential Risk and the Future of Humanity which addresses this issue, puts into question whether current public health and international conventions, and self-regulation by biotechnology companies and scientists are adequate.[144][145]
In the context of the 2019–2020 coronavirus pandemic Neal Baer writes that the "public, scientists, lawmakers, and others" "need to have thoughtful conversations about gene editing now".[146] Ensuring the biosafety level of laboratories may also be an important component of pandemic prevention. This issue may have gotten additional attention in 2020 after news outlets reported that U.S. State Department cables indicate that, although there may be no conclusive proof at the moment, the COVID-19 virus responsible for the COVID-19 pandemic may, possibly, have accidentally come from a Wuhan (China) laboratory, studying bat coronaviruses that included modifying virus genomes to enter human cells,[147][148] and determined to be unsafe by U.S. scientists in 2018, rather than from a natural source.[149][150][151] As of 18 May 2020, an official UN investigation into the origins of the COVID-19 virus, supported by over 120 countries, was being considered.[152] United States' president Donald Trump claimed to have seen evidence that gave him a "high degree of confidence" that the novel coronavirus originated in the Chinese laboratory but did not offer any evidence, data or details, contradicted statements by the United States' intelligence community and garnered a lot of harsh criticism and doubts.[153] As of 5 May, assessments and internal sources from the Five Eyes nations indicated that the coronavirus outbreak being the result of a laboratory accident was "highly unlikely", since the human infection was "highly likely" a result of natural human and animal interaction.[154] Many others have also criticized statements by US government officials and theories of laboratory release. Virologist and immunologist Vincent R. Racaniello said that "accident theories – and the lab-made theories before them – reflect a lack of understanding of the genetic make-up of Sars-CoV-2."[155] Virologist Peter Daszak stated that an estimated 1–7 million people in Southeast Asia who live or work in proximity to bats are infected each year with bat coronaviruses.[156] In January 2021, the WHO's investigations into the origin of COVID-19 was launched.[157][158] In early 2021, the hypothesis of a laboratory cause of the pandemic received renewed interest and expert consideration due to renewed media discussion.[159]
While biotechnology policies can substantially reduce the risk of a serious catastrophe, it may be important that relevant steps are initiated immediately and on a global basis.[40]
Dual-use knowledge and research
Martin Rees, author of the book Our Final Hour which also addresses this issue, states that while better understanding of viruses may allow for an improved capability to develop vaccines it may also lead to an increase in "the spread of 'dangerous knowledge' that would enable mavericks to make viruses more virulent and transmissible than they naturally are".[160] Different accelerations and priorizations of research may however be critical to pandemic prevention. A multitude of factors shape which knowledge about viruses with different use-cases, including vaccine-development, can be used by whom. Rees also states that "the global village will have its village idiots, and they will have global range".[161]
Experts have clarified that, for example, the definition of "dual use" is not well known and that the international community should better engage with the DIY bio community or biohacker students and in a way that does not stifle "localised innovation for peaceful purposes or people wanting to learn about biology".[29] As of 2022, only very few sophisticated centers could recreate SARS-CoV-2[41] However, for example the progress in genetic engineering enabled all the tools needed to create a virus to be "cheap, simple, and readily available".[162]
94% of countries have no national-level oversight measures for dual-use research.[40]
A biodefense expert cautions that overly strict rules "could prompt researchers to move their experiments to nations with less stringent oversight", suggesting there to be a need for international policies.[163]
Food markets and wild animal trade

In January 2020 during the SARS-CoV 2 outbreak experts in and outside China warned that wild animal markets, where the virus originated from, should be banned worldwide.[89][164] Some scientists point out that banning informal wet markets worldwide isn't the appropriate solution as fridges aren't available in many places and because much of the food for Africa and Asia is provided through such traditional markets. Some also caution that simple bans may force traders underground, where they may pay less attention to hygiene and some state that it's wild animals rather than farmed animals that are the natural hosts of many viruses.[46][47][118] Jonathan Kolby cautions about the risks and vulnerabilities present in the massive legal wildlife trade.[165]
Some traditional medicines (i.e. traditional African medicine, TCM) still use animal-based substances. Since these can trigger zoonosis,[166] a possible prevention could be changes to handbooks for practitioners of such traditional medicines (i.e. exclusion of animal-based substances). Senior adviser and veterinary epidemiologist at the National Food Institute at the Technical University of Denmark Ellis-Iversen states that in agricultural animal health "outbreaks of exotic disease in well-regulated countries rarely get big because we identify and control them right away".[47] New York City's Bronx Zoo's head veterinarian Paul Calle states that usually emerging infectious diseases from animals are the result from wildlife consumption and distribution on a commercial scale rather than a lone person hunting to feed their family.[47]
UN biodiversity chief, bipartisan lawmakers, experts and scientists have called for a global ban of wetmarkets and wildlife trade.[167][168][169][170] On January 26 China banned the trade of wild animals until the end of the coronavirus epidemic at the time.[171] On February 24 China announced a permanent ban on wildlife trade and consumption with some exceptions.[172] In early 2022 it was reported that the E.U. "is pushing for a global deal aimed at preventing new pandemics that could include a ban [a gradual shutdown] on wildlife markets".[81]
International coordination
The Global Health Security Agenda (GHSA) a network of countries, international organizations, NGOs and companies that aim to improve the world's ability to prevent, detect, and respond to infectious diseases. Sixty-seven countries have signed onto the GHSA framework.[173][174] Funding for the GHSA has been reduced since the launch in 2014, both in the US and globally.[115] The 194 WHO member states agreed in December 2021 to begin negotiations on the International Treaty on Pandemic Prevention, Preparedness and Response.[175][176][177] On the 2021 Global Health Summit, the G20 communicated to commit to promote a set of principles in the Rome Declaration.[178]
In a 2018 lecture in Boston Bill Gates called for a global effort to build a comprehensive pandemic preparedness and response system.[179][180] During the COVID-19 pandemic he called upon world leaders to "take what has been learned from this tragedy and invest in systems to prevent future outbreaks".[53] In a 2015 TED Talk he warned that "if anything kills over 10 million people in the next few decades, it's most likely to be a highly infectious virus rather than a war".[181] Numerous prominent, authoritative, expert or otherwise influential figures have similarly warned about elevated, underprepared or contemporary risks of pandemics and the need for efforts on an "international scale" long before 2015 and since at least 1988.[182][183] Later warnings include a 2015 study which concluded that "a potential risk of SARS-CoV re-emergence from viruses currently circulating in bat populations".[184]
Some have provided suggestions for organizational or coordinative preparedness for pandemic prevention including a mechanism by which many major economic powers pay into a global insurance fund which "could compensate a nation for economic losses if it acts quickly to close areas to trade and travel in order to stop a dangerous outbreak at its source"[185] or, similarly, sovereign or regional-level epidemic-insurance policies.[186] International collaboration including cooperative research and information-sharing has also been considered vital.[53]
As an example of domestic coordination, U.S. Senator Dianne Feinstein for the creation of a new interagency government entity, the Center for Combating Infectious Disease which would combine analytical and operational functions "to oversee all aspects of preventing, detecting, monitoring, and responding to major outbreaks such as coronavirus" and get provided with data and expertise by the Centers for Disease Control and Prevention.[47][187] The U.S. has also set up a "Global Zoonotic Disease Task Force" who would help "ensure an integrated approach to preventing, detecting, preparing for, and responding to zoonotic spillover".[188] However, "global preparedness is greater than the sum of national preparedness" and lacks concerted, collective and coordinated action.[189]
John Davenport advises to abandon widespread libertarian ideology which, according to him, "denies the importance of public goods or refuses to recognize their scope".[185] According to the CDC, investing in global health security and improving the organization's ability to prevent, detect, and respond to diseases could protect the health of American citizens as well as avert catastrophic costs.[190] Dennis Carroll argues for a "marriage" between scientific discovery and political decision-making and policy formulation.[46] Strengthened global governance – using facts, data, and science – with an emphasis on transparency and accountability[23] and independent monitoring are important.[189]
A study found there to be a need "for a renewed framework for global collective action that ensures conformity with international regulations and promotes effective prevention and response to pandemic infectious diseases" and recommended "greater authority for a global governing body, an improved ability to respond to pandemics, an objective evaluation system for national core public health capacities, more effective enforcement mechanisms, independent and sustainable funding, representativeness, and investment from multiple sectors, among others".[84]
Artificial induction of immunity and/or biocides
Outbreaks could be contained or delayed – to enable other containment-measures – or prevented by artificial induction of immunity and/or biocides in combination with other measures that include prediction or early detection of infectious human diseases.
Broad-spectrum antimicrobials, rapid antibody or drug development, and quick drug repurposing and medication provisioning may also be potential ways to prevent outbreak from becoming pandemics. In FY2016, DARPA initiated the Pandemic Prevention Platform (P3) program aimed at the "rapid discovery, testing, and manufacture of antibody treatments to fight any emerging disease threat".[191][192] The SARS-CoV-2 Omicron variant escaped the majority of existing SARS-CoV-2 neutralizing antibodies, including of sera from vaccinated and convalescent individuals.[193][194][195][196]
In a preprint published on March 24, 2020 researchers suggested that the unique transcriptional signature of SARS-CoV-2 in the human immune system may be responsible for the development of COVID-19: SARS-CoV-2 did not induce the antiviral genes that code for type I and type III interferons. This could be relevant for the development or repurposing of treatments.[197]
Vaccination
Development and provision of new vaccines usually takes years.[179] The Coalition for Epidemic Preparedness Innovations, which was launched in 2017, works on reducing the time of vaccine-development.[179] The Global Health Innovative Technology Fund (GHIT) is a public-private partnership fund which involves a national government, a UN agency, a consortium of pharmaceutical and diagnostics companies, and international philanthropic foundations to accelerate the creation of new vaccines, drugs and diagnostic tools for global health.[198][199] It is unclear whether vaccines can play a role in pandemic prevention alongside pandemic mitigation. Nathan Wolfe proposes that pathogen detection and prediction may allow establishing viral libraries before novel epidemics emerge – substantially decreasing the time to develop a new vaccine.[186] Public health surveillance expert and professor at Harvard University, John Brownstein says that "vaccines are still our main weapon".[200] Besides more rapid vaccine development and development that starts at the earliest possible time,[192] it may also be possible to develop more broader vaccines.[200] Misinformation and misconceptions about vaccines, including about their side-effects and (relative) risks, may be a problem.[200]
Food system- and livestock-specific measures
A review suggests that feeding the future human population would require increases in crop and animal production, albeit it is unclear which diets (e.g. future levels of meat production) they project. This would increase contact rates between humans and both wild and domestic animals and the use of antibiotics and thereby increase pandemic risks.[201] It also suggests that "since 1940, agricultural drivers were associated with >25% of all — and >50% of zoonotic — infectious diseases that emerged in humans".[201]
Moreover, selection for specific genes has made the animals genetically highly similar which could enable pathogens to spread more intensely among the livestock.[216]
A report by the FAIRR global investor network found that more than 70% of the biggest meat, fish and dairy producers were in danger of fostering future zoonotic pandemics due to lax safety standards, closely confined animals and the overuse of antibiotics.[217] Some have recommended food system-change, behaviour change,[47] different lifestyle choices and altered consumer spending including moving away from factory farming and towards more plant-based diets.[217][95][218]
Measures could include reducing meat production (except for potential cultured meat), de-intensifying livestock farming, reducing the use of antimicrobials, improving health and health-monitoring of livestock, increasing livestock biodiversity, further understanding of the genetic and functional basis of host adaptation,[219] improving occupational hygiene and safety of farming and food processing/food[201][216][141][73] (see also: HACCP and COVID-19 software (including contact tracing)).
Culling
Experts warned that depleting the numbers of species by culling to forestall human infections reduces genetic diversity and thereby puts future generations of the animals as well as people at risk while others contend that it's still the best, practical way to contain a virus of livestock.[220] There are also other problems with culling and there are alternatives to it, such as animal vaccination.[221][222] There are also problems with the forms of culling-implementation such as livestock producers and subsistence farmers who are unable to access compensation being motivated to conceal diseased animals rather than report them.[222]
Prevention versus mitigation
Pandemic prevention seeks to prevent pandemics while mitigation of pandemics seeks to reduce their severity and negative impacts. Some have called for a shift from a treatment-oriented society to a prevention-oriented one.[223] Authors of a 2010 study write that contemporary "global disease control focuses almost exclusively on responding to pandemics after they have already spread globally" and argue that the "wait-and-respond approach is not sufficient and that the development of systems to prevent novel pandemics before they are established should be considered imperative to human health".[224] Peter Daszak comments on the COVID-19 pandemic, saying "[t]he problem isn't that prevention was impossible, [i]t was very possible. But we didn't do it. Governments thought it was too expensive. Pharmaceutical companies operate for profit". The WHO reportedly had mostly neither the funding nor the power to enforce the large-scale global collaboration necessary to combat it.[225] Nathan Wolfe criticizes that "our current global public health strategies are reminiscent of cardiology in the 1950s when doctors focused solely on responding to heart attacks and ignored the whole idea of prevention".[87]
See also
- Antimicrobial resistance#Prevention
- Border control
- Travel during the COVID-19 pandemic – Restrictions by countries intended to stop spread of disease
- Globalization and disease – Overview of globalization and disease transmission
- Disease X – Placeholder infectious disease name from the WHO
- Global catastrophic risk – Potentially harmful worldwide events
- Global health
- Hazards of synthetic biology
- Health policy#Global health policy
- Infection control
- Molecular nanotechnology#Risks
- Pandemic treaty
- Pandemic predictions and preparations prior to COVID-19
- Biosecurity
- Priority-setting in global health
- Universal coronavirus vaccine
- Wastewater-based epidemiology
- Global Health Security Initiative
- Johns Hopkins Center for Health Security
- WHO Global Preparedness Monitoring Board
References
- Morens DM, Fauci AS (September 2020). "Emerging Pandemic Diseases: How We Got to COVID-19". Cell. 182 (5): 1077–1092. doi:10.1016/j.cell.2020.08.021. PMC 7428724. PMID 32846157.
- "WHO | SARS outbreak contained worldwide". WHO. Archived from the original on August 25, 2004.
- WHO. "SARS: How a global epidemic was stopped" (PDF). Retrieved 25 March 2020.
- "Middle East respiratory syndrome coronavirus (MERS-CoV)". www.who.int. Retrieved 18 February 2022.
- "Scientists evaluate zoonotic potential of NeoCoV, a coronavirus related to MERS-CoV". News-Medical.net. 30 January 2022. Retrieved 12 February 2022.
- Xiong, Qing; Cao, Lei; Ma, Chengbao; Liu, Chen; Si, Junyu; Liu, Peng; Gu, Mengxue; Wang, Chunli; Shi, Lulu; Tong, Fei; Huang, Meiling; Li, Jing; Zhao, Chufeng; Shen, Chao; Chen, Yu; Zhao, Huabin; Lan, Ke; Wang, Xiangxi; Yan, Huan (25 January 2022). "Close relatives of MERS-CoV in bats use ACE2 as their functional receptors". bioRxiv 10.1101/2022.01.24.477490.
- "NeoCov's potential danger to humans requires further study — WHO". TASS. Retrieved 12 February 2022.
- "Fact Check-NeoCov is not a new type of human transmissible coronavirus". Reuters. 1 February 2022.
- "Multi-country monkeypox outbreak in non-endemic countries". www.who.int. Retrieved 22 June 2022.
- Kozlov, Max (20 May 2022). "Monkeypox goes global: why scientists are on alert". Nature. 606 (7912): 15–16. doi:10.1038/d41586-022-01421-8. PMID 35595996. S2CID 248947652.
- "Monkeypox - United Kingdom of Great Britain and Northern Ireland". www.who.int. Retrieved 22 June 2022.
- Rigby, Jennifer; Roy, Mrinalika (24 May 2022). "WHO says monkeypox 'containable' as more govts start limited vaccinations". Reuters. Retrieved 22 June 2022.
- Cox, David. "Monkeypox Can Be Contained—but Time Is Running Out". Wired. Retrieved 22 June 2022.
- Titanji, Boghuma K; Tegomoh, Bryan; Nematollahi, Saman; Konomos, Michael; Kulkarni, Prathit A (1 July 2022). "Monkeypox: A Contemporary Review for Healthcare Professionals". Open Forum Infectious Diseases. 9 (7): ofac310. doi:10.1093/ofid/ofac310. ISSN 2328-8957. PMC 9307103. PMID 35891689.
- Group, World Bank (2014). The World Bank Group A to Z 2015. World Bank Publications. p. 119. ISBN 978-1-4648-0382-6. Retrieved 25 March 2020.
- Tolliver, Sandy (3 April 2020). "Want to stop pandemics? Strengthen public health systems in poor countries". TheHill. Retrieved 7 June 2020.
- Lu, Michael C. "What the world can do to halt future pandemics". Newsday. The Washington Post. Retrieved 5 June 2020.
- Sterzel, Eva (2006). "Pandemie-Prävention: Im Ernstfall Zeit gewinnen" [Pandemic prevention: save time in an emergency]. Nachrichten aus der Chemie. 54 (12): 1226–1227. doi:10.1002/nadc.20060541217. ISSN 1868-0054.
- Jackson, Mark (2016). The Routledge History of Disease. Routledge. p. 140. ISBN 978-1-134-85787-6. Retrieved 25 March 2020.
- "Pandemie-Bekämpfung Der nächste Ausbruch kommt bestimmt" [Pandemic control The next outbreak is sure to come]. Deutschlandfunk (in German). Retrieved 30 March 2020.
- Watts, Charlotte H.; Vallance, Patrick; Whitty, Christopher J. M. (18 February 2020). "Coronavirus: global solutions to prevent a pandemic". Nature. 578 (7795): 363. Bibcode:2020Natur.578R.363W. doi:10.1038/d41586-020-00457-y. PMID 32071448.
- "Promoting the Development of a Pandemic Risk Prevention and Monitoring System in Health Organizations for Post Covid-19 Restart" (PDF). PM World Journal. 2021.
- Blinken, Antony J.; Becerra, Xavier (5 October 2021). "Strengthening Global Health Security and Reforming the International Health Regulations: Making the World Safer From Future Pandemics". JAMA. 326 (13): 1255–1256. doi:10.1001/jama.2021.15611. ISSN 0098-7484. PMID 34464446. S2CID 237373952.
- Shehri, Saud Ali Al; Al-Sulaiman, A. M.; Azmi, Sarfuddin; Alshehri, Sultan S. (January 2022). "Bio-safety and bio-security: A major global concern for ongoing COVID-19 pandemic". Saudi Journal of Biological Sciences. 29 (1): 132–139. doi:10.1016/j.sjbs.2021.08.060. PMC 8404373. PMID 34483699.
- Hunger, Iris (July 2014). "Winning the battle against emerging pathogens". Bulletin of the Atomic Scientists. 70 (4): 22–25. Bibcode:2014BuAtS..70d..22H. doi:10.1177/0096340214539133. ISSN 0096-3402. S2CID 145732199.
- "Debating the transparency surrounding risky pathogen research". 30 January 2020.
- Willman, David; Muller, Madison. "A science in the shadows". Washington Post. Retrieved 6 June 2022.
- "The Challenges of Calculating a Lab Leak Risk". Undark Magazine. 1 June 2022.
- "The garage biohackers who manipulate DNA". Australian Financial Review. 23 September 2021. Retrieved 6 June 2022.
- Kerkhove, Maria D. Van; Ryan, Michael J.; Ghebreyesus, Tedros Adhanom (22 October 2021). "Preparing for "Disease X"". Science. 374 (6566): 377. Bibcode:2021Sci...374..377V. doi:10.1126/science.abm7796. PMID 34643114. S2CID 238746506.
- "Inside the risky bat-virus engineering that links America to Wuhan". MIT Technology Review. Retrieved 21 February 2022.
Two years later, Daszak and Shi published a paper reporting how the Chinese lab had engineered different versions of WIV1 and tested their infectiousness in human cells. The paper announced that the WIV had developed its own reverse-genetics system, following the Americans' lead. It also included a troubling detail: the work, which was funded in part by the NIH grant, had been done in a BSL-2 lab."
- Mourya, Devendra T.; Yadav, Pragya D.; Khare, Ajay; Khan, Anwar H. (October 2017). "Certification & validation of biosafety level-2 & biosafety level-3 laboratories in Indian settings & common issues". The Indian Journal of Medical Research. 146 (4): 459–467. doi:10.4103/ijmr.IJMR_974_16 (inactive 31 July 2022). PMC 5819027. PMID 29434059.
{{cite journal}}: CS1 maint: DOI inactive as of July 2022 (link) - Kojima, Kazunobu; Booth, Catherine Makison; Summermatter, Kathrin; Bennett, Allan; Heisz, Marianne; Blacksell, Stuart D.; McKinney, Michelle (20 April 2018). "Risk-based reboot for global lab biosafety". Science. 360 (6386): 260–262. Bibcode:2018Sci...360..260K. doi:10.1126/science.aar2231. PMID 29674576. S2CID 5046071.
- Lerner, Sharon (28 December 2021). "The Virus Hunters: How the Pursuit of Unknown Viruses Risks Triggering the Next Pandemic". The Intercept. Retrieved 12 February 2022.
- Mallapaty, Smriti (24 September 2021). "Closest known relatives of virus behind COVID-19 found in Laos". Nature. 597 (7878): 603. Bibcode:2021Natur.597..603M. doi:10.1038/d41586-021-02596-2. PMID 34561634. S2CID 237626322. Retrieved 20 October 2021.
- Temmam, Sarah; Vongphayloth, Khamsing; Salazar, Eduard Baquero; Munier, Sandie; Bonomi, Max; Régnault, Béatrice; Douangboubpha, Bounsavane; Karami, Yasaman; Chretien, Delphine; Sanamxay, Daosavanh; Xayaphet, Vilakhan; Paphaphanh, Phetphoumin; Lacoste, Vincent; Somlor, Somphavanh; Lakeomany, Khaithong; Phommavanh, Nothasin; Pérot, Philippe; Donati, Flora; Bigot, Thomas; Nilges, Michael; Rey, Félix; Werf, Sylvie van der; Brey, Paul; Eloit, Marc (17 September 2021). "Coronaviruses with a SARS-CoV-2-like receptor-binding domain allowing ACE2-mediated entry into human cells isolated from bats of Indochinese peninsula" (PDF). Research Square (Preprint). doi:10.21203/rs.3.rs-871965/v1. S2CID 237639577.
- Wu, Zhiqiang; Han, Yelin; Wang, Yuyang; Liu, Bo; Zhao, Lamei; Zhang, Junpeng; Su, Hao-Xiang; Zhao, Wenliang; Liu, Liguo; Bai, Shibin; Dong, Jie; Sun, Lilian; Zhu, Yafang; Zhou, Siyu; Song, Yiping; Sui, Hongtao; Yang, Jian; Wang, Jianwei; Zhang, Shuyi; Qian, Zhaohui; Jin, Qi (20 September 2021). "A comprehensive survey of bat sarbecoviruses across China for the origin tracing of SARS-CoV and SARS-CoV-2". Research Square (Preprint). doi:10.21203/rs.3.rs-885194/v1. S2CID 240599325.
- Patlovich, Scott J.; Emery, Robert J.; Whitehead, Lawrence W.; Brown, Eric L.; Flores, Rene (March 2015). "Assessing the Biological Safety Profession's Evaluation and Control of Risks Associated with the Field Collection of Potentially Infectious Specimens". Applied Biosafety. 20 (1): 27–40. doi:10.1177/153567601502000104. PMC 5760186. PMID 29326541.
- "FIELD BIORISK MANAGEMENT: AN ASSESSMENT OF THE BIOLOGICAL SAFETY PROFESSION" (PDF). Retrieved 6 June 2022.
- Piper, Kelsey (5 April 2022). "Why experts are terrified of a human-made pandemic — and what we can do to stop it". Vox. Retrieved 6 June 2022.
- "Biologists rush to re-create the China coronavirus from its genetic code". MIT Technology Review. Retrieved 6 June 2022.
- Ladner, Jason T. (29 September 2021). "Genomic signatures for predicting the zoonotic potential of novel viruses". PLOS Biology. 19 (9): e3001403. doi:10.1371/journal.pbio.3001403. PMC 8480851. PMID 34587150.
- Morse, Stephen S; Mazet, Jonna AK; Woolhouse, Mark; Parrish, Colin R; Carroll, Dennis; Karesh, William B; Zambrana-Torrelio, Carlos; Lipkin, W Ian; Daszak, Peter (1 December 2012). "Prediction and prevention of the next pandemic zoonosis". The Lancet. 380 (9857): 1956–1965. doi:10.1016/S0140-6736(12)61684-5. ISSN 0140-6736. PMC 3712877. PMID 23200504.
- Walsh, Bryan. "Virus Hunter: How One Scientist Is Preventing the Next Pandemic". Time. Retrieved 26 March 2020.
- McKie, Robin (24 June 2018). "Scientists aim to stop the devastation of Zika-like pandemics". The Observer. Retrieved 3 April 2020.
- "Before the Next Pandemic, an Ambitious Push to Catalog Viruses in Wildlife". Yale E360. Retrieved 8 June 2020.
- "To prevent pandemics, bridging the human and animal health divide". Salon. 1 June 2020. Retrieved 8 June 2020.
- Salama, Mostafa A.; Hassanien, Aboul Ella; Mostafa, Ahmad (13 May 2016). "The prediction of virus mutation using neural networks and rough set techniques". EURASIP Journal on Bioinformatics and Systems Biology. 2016 (1): 10. doi:10.1186/s13637-016-0042-0. ISSN 1687-4145. PMC 4867776. PMID 27257410.
- "Predicting the evolution of genetic mutations". phys.org. Retrieved 16 May 2020.
- Zhou, Juannan; McCandlish, David M. (14 April 2020). "Minimum epistasis interpolation for sequence-function relationships". Nature Communications. 11 (1): 1782. Bibcode:2020NatCo..11.1782Z. doi:10.1038/s41467-020-15512-5. ISSN 2041-1723. PMC 7156698. PMID 32286265.
- "AI may predict the next virus to jump from animals to humans". Public Library of Science. Retrieved 19 October 2021.
- Mollentze, Nardus; Babayan, Simon A.; Streicker, Daniel G. (28 September 2021). "Identifying and prioritizing potential human-infecting viruses from their genome sequences". PLOS Biology. 19 (9): e3001390. doi:10.1371/journal.pbio.3001390. ISSN 1545-7885. PMC 8478193. PMID 34582436.
- Kempe, Frederick (16 May 2020). "Op-ed: U.S. should enlist tech companies to build global quick response system to prevent future pandemic". CNBC. Retrieved 7 June 2020.
- McNeil, Donald G. Jr. (25 October 2019). "Scientists Were Hunting for the Next Ebola. Now the U.S. Has Cut Off Their Funding". The New York Times. Retrieved 25 March 2020.
- Sun, Lena H. "CDC to cut by 80 percent efforts to prevent global disease outbreak". Washington Post. Retrieved 26 March 2020.
- Pelley, Lauren. "Supercomputer helps Canadian researcher uncover thousands of viruses that could cause human diseases". Retrieved 12 February 2022.
- Edgar, Robert C.; Taylor, Jeff; Lin, Victor; Altman, Tomer; Barbera, Pierre; Meleshko, Dmitry; Lohr, Dan; Novakovsky, Gherman; Buchfink, Benjamin; Al-Shayeb, Basem; Banfield, Jillian F.; de la Peña, Marcos; Korobeynikov, Anton; Chikhi, Rayan; Babaian, Artem (February 2022). "Petabase-scale sequence alignment catalyses viral discovery". Nature. 602 (7895): 142–147. Bibcode:2022Natur.602..142E. doi:10.1038/s41586-021-04332-2. ISSN 1476-4687. PMID 35082445. S2CID 246297430.
- The Economist, April 4th 2020, page 14.
- Levy, Steven. "Could Crispr Be Humanity's Next Virus Killer?". Wired. Retrieved 25 March 2020.
- Abbott, Timothy R.; Dhamdhere, Girija; Liu, Yanxia; Lin, Xueqiu; Goudy, Laine; Zeng, Leiping; Chemparathy, Augustine; Chmura, Stephen; Heaton, Nicholas S.; Debs, Robert; Pande, Tara; Endy, Drew; Russa, Marie La; Lewis, David B.; Qi, Lei S. (14 March 2020). "Development of CRISPR as a prophylactic strategy to combat novel coronavirus and influenza". bioRxiv 10.1101/2020.03.13.991307.
- Nguyen, Tuan M.; Zhang, Yang; Pandolfi, Pier Paolo (March 2020). "Virus against virus: a potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses". Cell Research. 30 (3): 189–190. doi:10.1038/s41422-020-0290-0. PMC 7054296. PMID 32071427.
- Lewis, Tanya (23 October 2019). "Scientists Program CRISPR to Fight Viruses in Human Cells". Scientific American. Retrieved 1 April 2020.
- "Combatting Viruses with RNA-Targeted CRISPR". The Scientist Magazine®. Retrieved 1 April 2020.
- "New kind of CRISPR technology to target RNA, including RNA viruses like coronavirus". phys.org. Retrieved 3 April 2020.
- Wessels, Hans-Hermann; Méndez-Mancilla, Alejandro; Guo, Xinyi; Legut, Mateusz; Daniloski, Zharko; Sanjana, Neville E. (16 March 2020). "Massively parallel Cas13 screens reveal principles for guide RNA design". Nature Biotechnology. 38 (6): 722–727. doi:10.1038/s41587-020-0456-9. ISSN 1546-1696. PMC 7294996. PMID 32518401.
- "Researchers crack COVID-19 genome signature". phys.org. Retrieved 18 May 2020.
- Randhawa, Gurjit S.; Soltysiak, Maximillian P. M.; Roz, Hadi El; Souza, Camila P. E. de; Hill, Kathleen A.; Kari, Lila (24 April 2020). "Machine learning using intrinsic genomic signatures for rapid classification of novel pathogens: COVID-19 case study". PLOS ONE. 15 (4): e0232391. Bibcode:2020PLoSO..1532391R. doi:10.1371/journal.pone.0232391. ISSN 1932-6203. PMC 7182198. PMID 32330208.
- Souf, Selma (1 January 2016). "Recent advances in diagnostic testing for viral infections". Bioscience Horizons. 9. doi:10.1093/biohorizons/hzw010. Retrieved 26 March 2020.
- Tang, Patrick; Chiu, Charles (1 February 2010). "Metagenomics for the discovery of novel human viruses". Future Microbiology. 5 (2): 177–189. doi:10.2217/fmb.09.120. ISSN 1746-0913. PMID 20143943.
- Bearinger, Jane P.; Dugan, Lawrence C.; Baker, Brian R.; Hall, Sara B.; Ebert, Katja; Mioulet, Valerie; Madi, Mikidache; King, Donald P. (March 2011). "Development and Initial Results of a Low Cost, Disposable, Point-of-Care Testing Device for Pathogen Detection". IEEE Transactions on Biomedical Engineering. 58 (3): 805–808. doi:10.1109/TBME.2010.2089054. ISSN 1558-2531. PMC 3071014. PMID 21342806.
- Ye, Jiarong; Yeh, Yin-Ting; Xue, Yuan; Wang, Ziyang; Zhang, Na; Liu, He; Zhang, Kunyan; Ricker, RyeAnne; Yu, Zhuohang; Roder, Allison; Perea Lopez, Nestor; Organtini, Lindsey; Greene, Wallace; Hafenstein, Susan; Lu, Huaguang; Ghedin, Elodie; Terrones, Mauricio; Huang, Shengxi; Huang, Sharon Xiaolei (7 June 2022). "Accurate virus identification with interpretable Raman signatures by machine learning". Proceedings of the National Academy of Sciences. 119 (23): e2118836119. arXiv:2206.02788. Bibcode:2022PNAS..11918836Y. doi:10.1073/pnas.2118836119. PMC 9191668. PMID 35653572.
- Lee, Vernon J; Aguilera, Ximena; Heymann, David; Wilder-Smith, Annelies; Lee, Vernon J.; Aguilera, Ximena; Heymann, David L.; Wilder-Smith, Annelies; Bausch, Daniel G.; Briand, Sylvie; Bruschke, Christianne; Carmo, Eduardo H.; Cleghorn, Sean; Dandona, Lalit; Donnelly, Christl; Fall, Ibrahima Socé; Halton, Jane; Hatchett, Richard; Hong, Felicia; Horby, Peter; Ihekweazu, Chikwe; Jacobs, Michael; Khan, Kamran; Lin, Yijun; Leung, Gabriel; Low, Constance; McDonald, Bethan F.; Memish, Ziad A.; Morhard, Ryan; Ng, Deborah HL; Nkengasong, John; Pang, Junxiong; Redd, Stephen C.; Tan, Karen; Yeo, Wen Qing (January 2020). "Preparedness for emerging epidemic threats: a Lancet Infectious Diseases Commission". The Lancet Infectious Diseases. 20 (1): 17–19. doi:10.1016/S1473-3099(19)30674-7. PMC 7158988. PMID 31876487.
- Aiyar, Anaka; Pingali, Prabhu (1 August 2020). "Pandemics and food systems - towards a proactive food safety approach to disease prevention & management". Food Security. 12 (4): 749–756. doi:10.1007/s12571-020-01074-3. ISSN 1876-4525. PMC 7351553. PMID 32837645.
- Velavan, Thirumalaisamy P.; Meyer, Christian G. (July 2022). "Monkeypox 2022 outbreak: An update". Tropical Medicine & International Health. 27 (7): 604–605. doi:10.1111/tmi.13785. ISSN 1360-2276. PMID 35633308. S2CID 249128882.
- Zumla, Alimuddin; Alagaili, Abdulaziz N.; Cotten, Matthew; Azhar, Esam I. (7 September 2016). "Infectious diseases epidemic threats and mass gatherings: refocusing global attention on the continuing spread of the Middle East Respiratory syndrome coronavirus (MERS-CoV)". BMC Medicine. 14 (1): 132. doi:10.1186/s12916-016-0686-3. ISSN 1741-7015. PMC 5015245. PMID 27604081.
- Dambeck, Holger (28 April 2009). "Pandemie-Prävention: Experten gegen Wärmescanner auf Flughäfe" [Pandemic prevention: experts against heat scanners at airports]. DER SPIEGEL (in German). Retrieved 30 March 2020.
- "Harvard Launches International Scientific Task Force to Prevent Pandemics at the Source". C-CHANGE | Harvard T.H. Chan School of Public Health. 20 May 2021. Retrieved 6 June 2022. "Preventing Pandemics at the Source". C-CHANGE | Harvard T.H. Chan School of Public Health. 30 June 2021. Retrieved 6 June 2022.
- "InfectControl 2020 - InfectControl 2020". www.infectcontrol.de. Retrieved 1 April 2020.
- "Hygiene durch Architektur statt Antibiotika" [Hygiene through architecture instead of antibiotics]. Medizin Aspekte (in German). 1 April 2020. Retrieved 1 April 2020.
- "Pandemie-Prävention am Flughafen" [Pandemic prevention at the airport]. Fraunhofer-Gesellschaft (in German). Retrieved 30 March 2020.
- Guarascio, Francesco (9 February 2022). "Exclusive: EU wants pandemic treaty to ban wildlife markets, reward virus detection - source". Reuters. Retrieved 6 June 2022.
- Xing, Wanli; Wang, Jiadao; Zhao, Chao; Wang, Han; Bai, Liang; Pan, Liangbin; Li, Hang; Wang, Huili; Zhang, Zhi; Lu, Ying; Chen, Xiang; Shan, Sisi; Wang, Dong; Pan, Yifei; Weng, Ding; Zhou, Xinying; Huang, Rudan; He, Jianxing; Jin, Ronghua; Li, Weimin; Shang, Hong; Zhong, Nanshan; Cheng, Jing (1 April 2021). "A Highly Automated Mobile Laboratory for On-site Molecular Diagnostics in the COVID-19 Pandemic". Clinical Chemistry. 67 (4): 672–683. doi:10.1093/clinchem/hvab027. ISSN 0009-9147. PMC 8083610. PMID 33788940.
- Fall, Cheikh; Cappuyns, Aurélie; Faye, Oumar; Pauwels, Steven; Fall, Gamou; Dia, Ndongo; Diagne, Moussa M.; Diagne, Cheikh T.; Niang, Makhtar; Mbengue, Alassane; Faye, Martin; Dieng, Idrissa; Gningue, Babacar; Bousso, Abdoulaye; Faye, Ousmane; Pauwels, Rudi; Sall, Amadou A. (2020). "Field evaluation of a mobile biosafety laboratory in Senegal to strengthen rapid disease outbreak response and monitoring". African Journal of Laboratory Medicine. 9 (2): 1041. doi:10.4102/ajlm.v9i2.1041. ISSN 2225-2010. PMC 7479379. PMID 32934915.
- Duff, Johnathan H; Liu, Anicca; Saavedra, Jorge; Batycki, Jacob N; Morancy, Kendra; Stocking, Barbara; Gostin, Lawrence O; Galea, Sandro; Bertozzi, Stefano; Zuniga, Jose M; Alberto-Banatin, Carmencita; Dansua, Akua Sena; del Rio, Carlos; Kulzhanov, Maksut; Lee, Kelley; Scaglia, Gisela; Shahpar, Cyrus; Ullmann, Andrew J; Hoffman, Steven J; Weinstein, Michael; Szapocznik, José (June 2021). "A global public health convention for the 21st century". The Lancet Public Health. 6 (6): e428–e433. doi:10.1016/S2468-2667(21)00070-0. PMC 8099565. PMID 33964227. S2CID 233744547.
- Yen, Catherine; Hyde, Terri B; Costa, Alejandro J; Fernandez, Katya; Tam, John S; Hugonnet, Stéphane; Huvos, Anne M; Duclos, Philippe; Dietz, Vance J; Burkholder, Brenton T (March 2015). "The development of global vaccine stockpiles". The Lancet Infectious Diseases. 15 (3): 340–347. doi:10.1016/S1473-3099(14)70999-5. PMC 4712379. PMID 25661473.
- Kis, Zoltán; Kontoravdi, Cleo; Dey, Antu K.; Shattock, Robin; Shah, Nilay (July 2020). "Rapid development and deployment of high‐volume vaccines for pandemic response". Journal of Advanced Manufacturing and Processing. 2 (3): e10060. doi:10.1002/amp2.10060. ISSN 2637-403X. PMC 7361221. PMID 33977274.
- Wolfe, Nathan (29 April 2009). "Opinion | How to Prevent a Pandemic". The New York Times. Retrieved 25 March 2020.
- Loh, Elizabeth H.; Zambrana-Torrelio, Carlos; Olival, Kevin J.; Bogich, Tiffany L.; Johnson, Christine K.; Mazet, Jonna A. K.; Karesh, William; Daszak, Peter (1 July 2015). "Targeting Transmission Pathways for Emerging Zoonotic Disease Surveillance and Control". Vector Borne and Zoonotic Diseases. 15 (7): 432–437. doi:10.1089/vbz.2013.1563. ISSN 1530-3667. PMC 4507309. PMID 26186515.
- Carrington, Damian (25 March 2020). "Coronavirus: 'Nature is sending us a message', says UN environment chief". The Guardian. Retrieved 25 March 2020.
- Taylor, L. H.; Latham, S. M.; Woolhouse, M. E. (29 July 2001). "Risk factors for human disease emergence". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 356 (1411): 983–989. doi:10.1098/rstb.2001.0888. ISSN 0962-8436. PMC 1088493. PMID 11516376.
- Rasmussen, Angela L.; Katze, Michael G. (11 May 2016). "Genomic Signatures of Emerging Viruses: A New Era of Systems Epidemiology". Cell Host & Microbe. 19 (5): 611–618. doi:10.1016/j.chom.2016.04.016. ISSN 1931-3128. PMC 7104983. PMID 27173929.
- "Pandemie-Prävention in Nigeria". www.umweltdialog.de. Retrieved 30 March 2020.
- "Official Website of SORMAS". sormasorg.helmholtz-hzi.de. Retrieved 30 March 2020.
- Lawler, Daniel; Tourne, Isabelle. "The age of outbreaks: Experts warn of more animal disease threats". medicalxpress.com. Retrieved 10 July 2022.
- "Spillover Warning: How We Can Prevent the Next Pandemic". Yale E360. Retrieved 8 June 2020.
- Sharma, Samidha. "We need an early-warning system to prevent pandemics like Covid-19: Inder Singh". The Economic Times. Retrieved 12 August 2021.
- Miller, Maureen. "The next pandemic is already happening – targeted disease surveillance can help prevent it". The Conversation. Retrieved 12 August 2021.
- "Syndromic Surveillance and Bioterrorism-related Epidemics". Medscape. Retrieved 12 August 2021.
- "Syndromic e-surveillance: averting livestock disease outbreaks, improving livelihoods". International Livestock Research Institute. 2 August 2021. Retrieved 12 August 2021.
- Zeitung, Süddeutsche. "WHO-Frühwarnzentrum für Pandemien in Berlin eingeweiht". Süddeutsche.de (in German). Retrieved 6 June 2022.
Das Zentrum soll unter anderem mithilfe von künstlicher Intelligenz Unmengen von Daten analysieren. Dabei geht es etwa um Tiergesundheit, ungewöhnliche Krankheiten bei Menschen, Verhaltensänderungen der Menschen, Klimawandelfolgen oder Bevölkerungsverschiebungen. So sollen Muster früh erkannt werden. Es soll Modelle entwickeln, damit Risiken frühzeitig erkannt und besser eingeschätzt werden können.
- "WHO to set up pandemic early warning center in Germany | DW | 05.05.2021". Deutsche Welle (www.dw.com). Retrieved 6 June 2022.
- Zimmer, Carl; Carey, Benedict (21 December 2020). "The U.K. Coronavirus Variant: What We Know". The New York Times. Retrieved 16 January 2021.
- "WHO | SARS-CoV-2 Variants". WHO. Archived from the original on December 31, 2020. Retrieved 16 January 2021.
- "Update On Covid-19 (18th December 2020) - SA Corona Virus Online Portal". SA Corona Virus Online Portal. Retrieved 16 January 2021.
- Carlson, Andrea Michelson, Hilary Brueck, Nicholas. "The unlikely prospect of a COVID-19 variant that outsmarts vaccines 'keeps me up at night,' CDC Director Rochelle Walensky says in The EIC Interview". Business Insider. Retrieved 12 August 2021.
- Hirotsu, Yosuke; Omata, Masao (November 2021). "SARS-CoV-2 B.1.1.7 lineage rapidly spreads and replaces R.1 lineage in Japan: Serial and stationary observation in a community". Infection, Genetics and Evolution. 95: 105088. doi:10.1016/j.meegid.2021.105088. PMC 8454025. PMID 34560289.
- Mercatelli, Daniele; Holding, Andrew N; Giorgi, Federico M (22 March 2021). "Web tools to fight pandemics: the COVID-19 experience". Briefings in Bioinformatics. 22 (2): 690–700. doi:10.1093/bib/bbaa261. ISSN 1467-5463. PMC 7665357. PMID 33057582.
The architecture of Nextstrain is well designed and responds to the need for a continual surveillance to prevent uncontrolled outbreaks.
- "Detecting novel SARS-CoV-2 variants in New York City wastewater". University of Missouri. Retrieved 10 March 2022.
- Smyth, Davida S.; Trujillo, Monica; Gregory, Devon A.; Cheung, Kristen; Gao, Anna; Graham, Maddie; Guan, Yue; Guldenpfennig, Caitlyn; Hoxie, Irene; Kannoly, Sherin; Kubota, Nanami; Lyddon, Terri D.; Markman, Michelle; Rushford, Clayton; San, Kaung Myat; Sompanya, Geena; Spagnolo, Fabrizio; Suarez, Reinier; Teixeiro, Emma; Daniels, Mark; Johnson, Marc C.; Dennehy, John J. (3 February 2022). "Tracking cryptic SARS-CoV-2 lineages detected in NYC wastewater". Nature Communications. 13 (1): 635. Bibcode:2022NatCo..13..635S. doi:10.1038/s41467-022-28246-3. ISSN 2041-1723. PMC 8813986. PMID 35115523.
- "WHO global genomic surveillance strategy for pathogens with pandemic and epidemic potential 2022-2032". www.who.int. Retrieved 6 June 2022.
- Inzaule, Seth C; Tessema, Sofonias K; Kebede, Yenew; Ogwell Ouma, Ahmed E; Nkengasong, John N (1 September 2021). "Genomic-informed pathogen surveillance in Africa: opportunities and challenges". The Lancet Infectious Diseases. 21 (9): e281–e289. doi:10.1016/S1473-3099(20)30939-7. ISSN 1473-3099. PMC 7906676. PMID 33587898.
- "'This May Not Be The Big One': Army Scientists Warn of Deadlier Pandemics to Come". Defense One. Retrieved 6 June 2022.
- Pike, Jamison; Bogich, Tiffany; Elwood, Sarah; Finnoff, David C.; Daszak, Peter (30 December 2014). "Economic optimization of a global strategy to address the pandemic threat". Proceedings of the National Academy of Sciences. 111 (52): 18519–18523. Bibcode:2014PNAS..11118519P. doi:10.1073/pnas.1412661112. ISSN 0027-8424. PMC 4284561. PMID 25512538.
- Cheng, Vincent C. C.; Lau, Susanna K. P.; Woo, Patrick C. Y.; Yuen, Kwok Yung (1 October 2007). "Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection". Clinical Microbiology Reviews. 20 (4): 660–694. doi:10.1128/CMR.00023-07. ISSN 0893-8512. PMC 2176051. PMID 17934078.
- Jenkins, Bonnie (27 March 2020). "Now is the time to revisit the Global Health Security Agenda". Brookings. Retrieved 1 April 2020.
- Cameron, Beth. "Perspective | I ran the White House pandemic office. Trump closed it". Washington Post. Retrieved 1 April 2020.
- Bernstein, Aaron S.; Ando, Amy W.; Loch-Temzelides, Ted; Vale, Mariana M.; Li, Binbin V.; Li, Hongying; Busch, Jonah; Chapman, Colin A.; Kinnaird, Margaret; Nowak, Katarzyna; Castro, Marcia C.; Zambrana-Torrelio, Carlos; Ahumada, Jorge A.; Xiao, Lingyun; Roehrdanz, Patrick; Kaufman, Les; Hannah, Lee; Daszak, Peter; Pimm, Stuart L.; Dobson, Andrew P. (4 February 2022). "The costs and benefits of primary prevention of zoonotic pandemics". Science Advances. 8 (5): eabl4183. Bibcode:2022SciA....8.4183B. doi:10.1126/sciadv.abl4183. ISSN 2375-2548. PMC 8816336. PMID 35119921.
- Vidal, John (18 March 2020). "'Tip of the iceberg': is our destruction of nature responsible for Covid-19?". The Guardian. Retrieved 28 March 2020.
- "WHO | Climate change and human health - risks and responses. Summary". WHO. Archived from the original on December 23, 2009. Retrieved 27 March 2020.
- Carlson, Colin J.; Albery, Gregory F.; Merow, Cory; Trisos, Christopher H.; Zipfel, Casey M.; Eskew, Evan A.; Olival, Kevin J.; Ross, Noam; Bansal, Shweta (28 April 2022). "Climate change increases cross-species viral transmission risk". Nature. 607 (7919): 555–562. bioRxiv 10.1101/2020.01.24.918755. doi:10.1038/s41586-022-04788-w. ISSN 1476-4687. PMID 35483403. S2CID 248430532.
- News article: Zimmer, Carl (28 April 2022). "Climate Change Will Accelerate Viral Spillovers, Study Finds". The New York Times. Retrieved 13 May 2022.
- Wu, Xiaoxu; Lu, Yongmei; Zhou, Sen; Chen, Lifan; Xu, Bing (1 January 2016). "Impact of climate change on human infectious diseases: Empirical evidence and human adaptation". Environment International. 86: 14–23. doi:10.1016/j.envint.2015.09.007. ISSN 0160-4120. PMID 26479830.
- "Could ancient viruses from melting permafrost cause the next pandemic?". New Scientist. Retrieved 6 June 2022.
- "Biggest-ever virus revived from Stone Age permafrost". New Scientist. Retrieved 6 June 2022.
- "BBC Earth | Home". Retrieved 6 June 2022.
- Yirka, Bob. "Bacteria species found in glacial ice could pose disease risk as glaciers melt from global warming". phys.org. Retrieved 15 July 2022.
- Liu, Yongqin; Ji, Mukan; Yu, Tao; Zaugg, Julian; Anesio, Alexandre M.; Zhang, Zhihao; Hu, Songnian; Hugenholtz, Philip; Liu, Keshao; Liu, Pengfei; Chen, Yuying; Luo, Yingfeng; Yao, Tandong (27 June 2022). "A genome and gene catalog of glacier microbiomes". Nature Biotechnology: 1–8. doi:10.1038/s41587-022-01367-2. ISSN 1546-1696. PMID 35760913. S2CID 250091380.
- "How Forest Loss Is Leading To a Rise in Human Disease". Yale E360. Retrieved 27 March 2020.
- "Deforestation is leading to more infectious diseases in humans". Science. 22 November 2019. Retrieved 27 March 2020.
- Olivero, Jesús; Fa, John E.; Real, Raimundo; Márquez, Ana L.; Farfán, Miguel A.; Vargas, J. Mario; Gaveau, David; Salim, Mohammad A.; Park, Douglas; Suter, Jamison; King, Shona; Leendertz, Siv Aina; Sheil, Douglas; Nasi, Robert (30 October 2017). "Recent loss of closed forests is associated with Ebola virus disease outbreaks". Scientific Reports. 7 (1): 14291. Bibcode:2017NatSR...714291O. doi:10.1038/s41598-017-14727-9. ISSN 2045-2322. PMC 5662765. PMID 29085050.
- Sehgal, R. N. M. (15 March 2010). "Deforestation and avian infectious diseases". The Journal of Experimental Biology. 213 (6): 955–960. doi:10.1242/jeb.037663. ISSN 0022-0949. PMC 2829318. PMID 20190120.
- Bloomfield, Laura S. P.; McIntosh, Tyler L.; Lambin, Eric F. (1 April 2020). "Habitat fragmentation, livelihood behaviors, and contact between people and nonhuman primates in Africa". Landscape Ecology. 35 (4): 985–1000. doi:10.1007/s10980-020-00995-w. ISSN 1572-9761. S2CID 214731443.
- Vidal, John (2020-03-18). "'Tip of the iceberg': is our destruction of nature responsible for Covid-19?". The Guardian. ISSN 0261-3077. Retrieved 2020-11-10.
- "Deadly diseases from wildlife thrive when nature is destroyed, study finds". the Guardian. 2020-08-05. Retrieved 2020-11-10.
- Fisher, Judith Lorraine; Woolaston, Katie. "UN report says up to 850,000 animal viruses could be caught by humans, unless we protect nature". The Conversation. Retrieved 2020-11-10.
- "Stanford: How Humanity Has 'Engineered a World Ripe for Pandemics'". SciTechDaily. 28 March 2020. Retrieved 3 April 2020.
- Biodiversity loss and the ecology of infectious disease
- The best way to avoid future pandemics? Protect the natural world
- Beyond exclusion: alternative approaches to biodiversity conservation in the developing tropics
- "COVID-19 and nature are linked. So should be the recovery". World Economic Forum. Retrieved 5 June 2020.
- Everard, Mark; Johnston, Paul; Santillo, David; Staddon, Chad (1 September 2020). "The role of ecosystems in mitigation and management of Covid-19 and other zoonoses". Environmental Science & Policy. 111: 7–17. doi:10.1016/j.envsci.2020.05.017. ISSN 1462-9011. PMC 7247996. PMID 32501392.
- Greger, Michael (September 2021). "Primary Pandemic Prevention". American Journal of Lifestyle Medicine. 15 (5): 498–505. doi:10.1177/15598276211008134. PMC 8504329. PMID 34646097.
- Johnson, Christine K.; Hitchens, Peta L.; Pandit, Pranav S.; Rushmore, Julie; Evans, Tierra Smiley; Young, Cristin C. W.; Doyle, Megan M. (8 April 2020). "Global shifts in mammalian population trends reveal key predictors of virus spillover risk". Proceedings of the Royal Society B: Biological Sciences. 287 (1924): 20192736. doi:10.1098/rspb.2019.2736. PMC 7209068. PMID 32259475.
- UNEP Frontiers 2016 Report: Emerging Issues of Environmental Concern (PDF). Nairoby: United Nations Environment Programme. 2016. pp. 18–32. ISBN 978-92-807-3553-6. Retrieved 1 May 2020.
 Text is available under a Creative Commons Attribution 4.0 International License
Text is available under a Creative Commons Attribution 4.0 International License - Ord, Toby (6 March 2020). "Why we need worst-case thinking to prevent pandemics". The Guardian. Retrieved 1 April 2020.
- Ord, Toby (2021-03-23). "Covid-19 has shown humanity how close we are to the edge". The Guardian. Retrieved 2021-03-26.
- "Could a rogue scientist use CRISPR to conjure another pandemic?". STAT. 26 March 2020. Retrieved 27 March 2020.
- Yang, Yang; et al. (June 10, 2015). "Two Mutations Were Critical for Bat-to-Human Transmission of Middle East Respiratory Syndrome Coronavirus". Journal of Virology. 89 (17): 9119–9123. doi:10.1128/JVI.01279-15. PMC 4524054. PMID 26063432.
- Chen, Stephen (6 February 2020). "Coronavirus: bat scientist's cave exploits offer hope to beat virus 'sneakier than Sars' - Shi Zhengli is one of the scores of scientists joining a global effort to hunt down the new coronavirus - But some people have blamed her for creating it in the first place". South China Morning Post. Retrieved 15 April 2020.
- Rogin, Josh (14 April 2020). "State Department cables warned of safety issues at Wuhan lab studying bat coronaviruses". The Washington Post. Retrieved 15 April 2020.
- Campbell, Josh; Atwood, Kylie; Perez, Evan (16 April 2020). "US explores possibility that coronavirus spread started in Chinese lab, not a market". CNN News. Retrieved 16 April 2020.
- Rincon, Paul (16 April 2020). "Coronavirus: Is there any evidence for lab release theory?". BBC News. Retrieved 17 April 2020.
- Porter, Tom (18 May 2020). "More than 120 countries are backing a UN motion to investigate the origins of the coronavirus, despite China's objections". Business Insider. Retrieved 18 May 2020.
- "Trump contradicts US intel community by claiming he's seen evidence coronavirus originated in Chinese lab". CNN. Retrieved 7 June 2020.
- Marquardt, Alex; Atwood, Kylie; Cohen, Zachary (5 May 2020). "Intel shared among US allies indicates virus outbreak more likely came from market, not a Chinese lab". CNN. Retrieved 7 May 2020.
- McCarthy S, Chen S (11 April 2020). "Bat virus? Bioweapon? What the science says about Covid-19 origins". South China Morning Post.
- Barclay, Eliza (23 April 2020). "Why these scientists still doubt the coronavirus leaked from a Chinese lab". Vox.
- Nebehay, Stephanie (18 January 2021). "U.S. and China clash at WHO over scientific mission in Wuhan". Reuters. Archived from the original on 18 January 2021. Retrieved 18 January 2021.
- Fujiyama, Emily Wang (28 January 2021). "WHO team in Wuhan departs quarantine for COVID origins study". AP News. Archived from the original on 11 February 2021. Retrieved 12 February 2021.
- Knight, Peter (21 June 2021). "COVID-19: Why lab-leak theory is back despite little new evidence". The Conversation. Melbourne. Archived from the original on 18 July 2021. Retrieved 18 July 2021.
- Overbye, Dennis (2 June 2020). "Going Viral, or Not, in the Milky Way". The New York Times. Retrieved 7 June 2020.
- "Coronavirus: 'Recipe for instability', says futurist who predicted extinction event". The National. 2 June 2020. Retrieved 7 June 2020.
- Wadhwa, Vivek. "The Genetic Engineering Genie Is Out of the Bottle". Foreign Policy. Retrieved 6 June 2022.
- "Spurred by pandemic, U.S. government will revisit federal policies on risky virus research". www.science.org. Retrieved 6 June 2022.
- Boseley, Sarah (24 January 2020). "Calls for global ban on wild animal markets amid coronavirus outbreak". The Guardian. Retrieved 25 March 2020.
- "To prevent the next pandemic, it's the legal wildlife trade we should worry about". NationalGeographic. 7 May 2020. Retrieved 5 June 2020.
- Africa's growing risk of diseases that spread from animals to people
- "Experts call for global ban on live animal markets, wildlife trade amidst coronavirus outbreak". CBC. Retrieved 5 June 2020.
- Greenfield, Patrick (6 April 2020). "Ban wildlife markets to avert pandemics, says UN biodiversity chief". The Guardian. Retrieved 5 June 2020.
- Wise, Justin (9 April 2020). "Bipartisan lawmakers call for global 'wet markets' ban amid coronavirus crisis". TheHill. Retrieved 5 June 2020.
- Ceballos, Gerardo; Ehrlich, Paul R.; Raven, Peter H. (June 1, 2020). "Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction". PNAS. 117 (24): 13596–13602. Bibcode:2020PNAS..11713596C. doi:10.1073/pnas.1922686117. PMC 7306750. PMID 32482862.
The horrific coronavirus disease 2019 (Covid-19) pandemic that we are experiencing, of which we still do not fully understand the likely economic, political, and social global impacts, is linked to wildlife trade. It is imperative that wildlife trade for human consumption is considered a gigantic threat to both human health and wildlife conservation. Therefore, it has to be completely banned, and the ban strictly enforced, especially in China, Vietnam, Indonesia, and other countries in Asia
- Denyer, Simon. "China bans wild animal trade until coronavirus epidemic is eliminated". Washington Post. Retrieved 25 March 2020.
- Gorman, James (27 February 2020). "China's Ban on Wildlife Trade a Big Step, but Has Loopholes, Conservationists Say". The New York Times. Retrieved 25 March 2020.
- "CDC Global Health - CDC and the Global Health Security Agenda". www.cdc.gov. 19 February 2020. Retrieved 1 April 2020.
- "Global Health Security Agenda". Global Health Security Agenda. Retrieved 1 April 2020.
- "World Health Assembly agrees to launch process to develop historic global accord on pandemic prevention, preparedness and response". World Health Organization. 1 December 2021. Retrieved 2021-12-02.
- Cumming-Bruce, Nick (2021-12-01). "W.H.O. members agree to begin talks on a global pandemic treaty". The New York Times. ISSN 0362-4331. Retrieved 2021-12-02.
- Nebehay, Stephanie (2021-11-28). "WHO reaches draft consensus on future pandemic treaty". Reuters. Retrieved 2021-12-02.
- "Rome Declaration". global-health-summit.europa.eu. Retrieved 6 June 2022.
- Tindera, Michela. "Bill Gates Calls For, And Funds, Steps To Prevent A Global Pandemic". Forbes. Retrieved 1 April 2020.
- Gates, Bill. "The next epidemic is coming. Here's how we can make sure we're ready". gatesnotes.com. Retrieved 1 April 2020.
- "Bill Gates warned of a deadly pandemic for years — and said we wouldn't be ready to handle it". CBS. Retrieved 5 June 2020.
- Lederberg, Joshua (5 August 1988). "Medical Science, Infectious Disease, and the Unity of Humankind". JAMA. 260 (5): 684–685. doi:10.1001/jama.1988.03410050104039. ISSN 0098-7484. PMID 3392795. Retrieved 6 October 2020.
- Henig, Robin Marantz (8 April 2020). "Experts warned of a pandemic decades ago. Why weren't we ready?". National Geographic. Retrieved 6 October 2020.
- Menachery, Vineet D.; Yount, Boyd L.; Debbink, Kari; Agnihothram, Sudhakar; Gralinski, Lisa E.; Plante, Jessica A.; Graham, Rachel L.; Scobey, Trevor; Ge, Xing-Yi; Donaldson, Eric F.; Randell, Scott H.; Lanzavecchia, Antonio; Marasco, Wayne A.; Shi, Zhengli-Li; Baric, Ralph S. (December 2015). "A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence". Nature Medicine. 21 (12): 1508–1513. doi:10.1038/nm.3985. ISSN 1546-170X. PMC 4797993. PMID 26552008.
- "How an alliance of democracies can prevent future pandemics". Salon. 26 April 2020. Retrieved 5 June 2020.
- "COVID-19 Won't Be the Last Pandemic. Here's What We Can Do to Protect Ourselves". Time. Retrieved 5 June 2020.
- "Feinstein: The U.S. wasn't ready for coronavirus. We must learn from that". Los Angeles Times. 27 March 2020. Retrieved 8 June 2020.
- Coons, Christopher A. (20 May 2021). "S.1737 - 117th Congress (2021-2022): Global Pandemic Prevention and Biosecurity Act". www.congress.gov. Retrieved 6 June 2022.
- "The world was woefully unprepared for a pandemic. Let's be ready for the next one | Elhadj As Sy". The Guardian. 26 October 2021. Retrieved 6 June 2022.
- "Why It Matters: The Pandemic Threat | Division of Global Health Protection | Global Health | CDC". www.cdc.gov. 4 May 2020. Retrieved 5 June 2020.
- "Defense Advanced Research Projects – Pandemic Prevention Platform". Retrieved 21 February 2022.
- Franconi, Rosella; Illiano, Elena; Paolini, Francesca; Massa, Silvia; Venuti, Aldo; Demurtas, Olivia Costantina (2018). "Rapid and Low-Cost Tools Derived from Plants to Face Emerging/Re-emerging Infectious Diseases and Bioterrorism Agents". Defence Against Bioterrorism. NATO Science for Peace and Security Series A: Chemistry and Biology. Springer Netherlands: 123–139. doi:10.1007/978-94-024-1263-5_10. ISBN 978-94-024-1262-8. S2CID 169765240.
- Yunlong Cao; et al. (23 December 2021). "Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies". Nature. doi:10.1038/d41586-021-03796-6. S2CID 245455422.
- Wilhelm, Alexander; Widera, Marek; Grikscheit, Katharina; Toptan, Tuna; Schenk, Barbara; Pallas, Christiane; Metzler, Melinda; Kohmer, Niko; Hoehl, Sebastian; Helfritz, Fabian A.; Wolf, Timo; Goetsch, Udo; Ciesek, Sandra (8 December 2021). "Reduced Neutralization of SARS-CoV-2 Omicron Variant by Vaccine Sera and Monoclonal Antibodies". medRxiv. doi:10.1101/2021.12.07.21267432. S2CID 244950946.
- Liu, Lihong; Iketani, Sho; Guo, Yicheng; Chan, Jasper F-W.; Wang, Maple; Liu, Liyuan; Luo, Yang; Chu, Hin; Huang, Yiming; Nair, Manoj S.; Yu, Jian; Chik, Kenn K-H.; Yuen, Terrence T-T.; Yoon, Chaemin; To, Kelvin K-W.; Chen, Honglin; Yin, Michael T.; Sobieszczyk, Magdalena E.; Huang, Yaoxing; Wang, Harris H.; Sheng, Zizhang; Yuen, Kwok-Yung; Ho, David D. (23 December 2021). "Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2". Nature. 602 (7898): 676–681. doi:10.1038/d41586-021-03826-3. PMID 35016198. S2CID 245462866.
- Rössler, Annika; Riepler, Lydia; Bante, David; Laer, Dorothee von; Kimpel, Janine (11 December 2021). "SARS-CoV-2 B.1.1.529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals". medRxiv. doi:10.1101/2021.12.08.21267491. S2CID 245019954.
- "Cells' Response to SARS-CoV-2 Different from Flu, RSV". The Scientist Magazine®. Retrieved 1 April 2020.
- Slingsby, BT; Kurokawa, Kiyoshi (2013). "The Global Health Innovative Technology (GHIT) Fund: Financing medical innovations for neglected populations". The Lancet Global Health. 1 (4): e184–5. doi:10.1016/S2214-109X(13)70055-X. PMID 25104343.
- "Investing In Drugs That Won't Make Money", Forbes, April 30, 2015, accessed on 9/28/2015
- Guynup, Sharon. "Preparing for the Next Pandemic". Scientific American. Retrieved 8 June 2020.
- Rohr, Jason R.; Barrett, Christopher B.; Civitello, David J.; Craft, Meggan E.; Delius, Bryan; DeLeo, Giulio A.; Hudson, Peter J.; Jouanard, Nicolas; Nguyen, Karena H.; Ostfeld, Richard S.; Remais, Justin V.; Riveau, Gilles; Sokolow, Susanne H.; Tilman, David (June 2019). "Emerging human infectious diseases and the links to global food production". Nature Sustainability. 2 (6): 445–456. doi:10.1038/s41893-019-0293-3. ISSN 2398-9629. PMC 7091874. PMID 32219187.
- González, Neus; Marquès, Montse; Nadal, Martí; Domingo, José L. (November 1, 2020). "Meat consumption: Which are the current global risks? A review of recent (2010–2020) evidences". Food Research International. 137: 109341. doi:10.1016/j.foodres.2020.109341. ISSN 0963-9969. PMC 7256495. PMID 33233049.
- Alarcón, Laura Valeria; Allepuz, Alberto; Mateu, Enric (January 4, 2021). "Biosecurity in pig farms: a review". Porcine Health Management. 7 (1): 5. doi:10.1186/s40813-020-00181-z. ISSN 2055-5660. PMC 7780598. PMID 33397483.
- Greger, Michael (September 2021). "Primary Pandemic Prevention". American Journal of Lifestyle Medicine. 15 (5): 498–505. doi:10.1177/15598276211008134. ISSN 1559-8276. PMC 8504329. PMID 34646097.
- Yamaji, Reina; Saad, Magdi D.; Davis, Charles T.; Swayne, David E.; Wang, Dayan; Wong, Frank Y.K.; McCauley, John W.; Peiris, J.S. Malik; Webby, Richard J.; Fouchier, Ron A.M.; Kawaoka, Yoshihiro; Zhang, Wenqing (May 2020). "Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses". Reviews in Medical Virology. 30 (3): e2099. doi:10.1002/rmv.2099. ISSN 1052-9276. PMC 9285678. PMID 32135031. S2CID 212565735.
- MacMahon, Kathleen L.; Delaney, Lisa J.; Kullman, Greg; Gibbins, John D.; Decker, John; Kiefer, Max J. (May 2008). "Protecting Poultry Workers from Exposure to Avian Influenza Viruses". Public Health Reports. 123 (3): 316–322. doi:10.1177/003335490812300311. PMC 2289984. PMID 19006973.
- Shi, Weifeng; Gao, George F. (May 21, 2021). "Emerging H5N8 avian influenza viruses". Science. 372 (6544): 784–786. Bibcode:2021Sci...372..784S. doi:10.1126/science.abg6302. PMID 34016764. S2CID 234794580.
- Wignjadiputro, Ira; Widaningrum, Christina; Setiawaty, Vivi; Widuri Wulandari, Endang; Sihombing, Sinurtina; Prasetyo, Wing Ardi; Azhar, Muhammad; iL Rim, Kwang; Pang Junxiong, Vincent; Waworuntu, Wiendra; Subuh, Mohammad (July 1, 2020). "Whole–of–society approach for influenza pandemic epicenter Containment exercise in Indonesia". Journal of Infection and Public Health. 13 (7): 994–997. doi:10.1016/j.jiph.2019.12.009. ISSN 1876-0341. PMID 32122819. S2CID 211831987.
- "Meat-eating creates risk of new pandemic that 'would make Covid look like a dress rehearsal'". The Independent. January 30, 2021. Retrieved May 31, 2022.
- Sutton, Troy C. (September 2018). "The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses". Viruses. 10 (9): 461. doi:10.3390/v10090461. ISSN 1999-4915. PMC 6164301. PMID 30154345.
- Murray, Christopher JL; Ikuta, Kevin Shunji; Sharara, Fablina; Swetschinski, Lucien; Aguilar, Gisela Robles; Gray, Authia; Han, Chieh; Bisignano, Catherine; Rao, Puja; Wool, Eve; Johnson, Sarah C. (January 19, 2022). "Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis". The Lancet. 399 (10325): 629–655. doi:10.1016/S0140-6736(21)02724-0. ISSN 0140-6736. PMC 8841637. PMID 35065702. S2CID 246077406.
- Monger, Xavier C.; Gilbert, Alex-An; Saucier, Linda; Vincent, Antony T. (October 2021). "Antibiotic Resistance: From Pig to Meat". Antibiotics. 10 (10): 1209. doi:10.3390/antibiotics10101209. ISSN 2079-6382. PMC 8532907. PMID 34680790.
- Nüesch-Inderbinen, Magdalena; Treier, Andrea; Zurfluh, Katrin; Stephan, Roger (October 2019). "Raw meat-based diets for companion animals: a potential source of transmission of pathogenic and antimicrobial-resistant Enterobacteriaceae". Royal Society Open Science. 6 (10): 191170. Bibcode:2019RSOS....691170N. doi:10.1098/rsos.191170. PMC 6837177. PMID 31824726.
- Pandey, Santosh; Kalwa, Upender; Kong, Taejoon; Guo, Baoqing; Gauger, Phillip C.; Peters, David J.; Yoon, Kyoung-Jin (September 2021). "Behavioral Monitoring Tool for Pig Farmers: Ear Tag Sensors, Machine Intelligence, and Technology Adoption Roadmap". Animals. 11 (9): 2665. doi:10.3390/ani11092665. ISSN 2076-2615. PMC 8466302. PMID 34573631.
- Clifford, Katie; Desai, Darash; Prazeres da Costa, Clarissa; Meyer, Hannelore; Klohe, Katharina; Winkler, Andrea; Rahman, Tanvir; Islam, Taohidul; Zaman, Muhammad H (September 1, 2018). "Antimicrobial resistance in livestock and poor quality veterinary medicines". Bulletin of the World Health Organization. 96 (9): 662–664. doi:10.2471/BLT.18.209585. PMC 6154060. PMID 30262949.
- Samuel, Sigal (22 April 2020). "The meat we eat is a pandemic risk, too". Vox. Retrieved 6 June 2022.
- "Jane Goodall: humanity is finished if it fails to adapt after Covid-19". the Guardian. 3 June 2020. Retrieved 7 June 2020.
- "We Need to Rethink Our Food System to Prevent the Next Pandemic". Time. Retrieved 7 June 2020.
- Mourkas, Evangelos; Taylor, Aidan J.; Méric, Guillaume; Bayliss, Sion C.; Pascoe, Ben; Mageiros, Leonardos; Calland, Jessica K.; Hitchings, Matthew D.; Ridley, Anne; Vidal, Ana; Forbes, Ken J.; Strachan, Norval J. C.; Parker, Craig T.; Parkhill, Julian; Jolley, Keith A.; Cody, Alison J.; Maiden, Martin C. J.; Kelly, David J.; Sheppard, Samuel K. (19 May 2020). "Agricultural intensification and the evolution of host specialism in the enteric pathogen Campylobacter jejuni". Proceedings of the National Academy of Sciences. 117 (20): 11018–11028. doi:10.1073/pnas.1917168117. ISSN 0027-8424. PMC 7245135. PMID 32366649.
- Waltz, Emily (1 June 2006). "Pandemic prevention schemes threaten diversity, experts warn". Nature Medicine. 12 (6): 598. doi:10.1038/nm0606-598a. PMID 16760992. S2CID 1145076. Retrieved 25 March 2020.
- Butler, Declan (1 April 2005). "Vaccination will work better than culling, say bird flu experts". Nature. 434 (7035): 810. Bibcode:2005Natur.434..810B. doi:10.1038/4344810a. ISSN 1476-4687. PMID 15829925. S2CID 4347170.
- Blackburn, Christine Crudo; Natsios, Andrew S.; Parker, Gerald W. Jr.; Katz, Rebecca; Osterholm, Michael T.; Laine, Glen A.; Fair, Joseph (May 2018). "Global leadership at the crossroads: Are we prepared for the next pandemic?". Scowcroft Institute of International Affairs (The Bush School).
- Manika, D.; Golden, L. (2011). "Self-efficacy, Threat, Knowledge, and Information Receptivity: Exploring Pandemic Prevention Behaviors to Enhance Societal Welfare". Academy of Health Care Management Journal. Retrieved 25 March 2020.
- Hughes, James M.; Wilson, Mary E.; Pike, Brian L.; Saylors, Karen E.; Fair, Joseph N.; LeBreton, Matthew; Tamoufe, Ubald; Djoko, Cyrille F.; Rimoin, Anne W.; Wolfe, Nathan D. (15 June 2010). "The Origin and Prevention of Pandemics". Clinical Infectious Diseases. 50 (12): 1636–1640. doi:10.1086/652860. ISSN 1058-4838. PMC 2874076. PMID 20450416.
- Kahn, Jennifer (21 April 2020). "How Scientists Could Stop the Next Pandemic Before It Starts". The New York Times. Retrieved 8 June 2020.