Thiocarlide
Thiocarlide (or tiocarlide or isoxyl) is a thiourea drug used in the treatment of tuberculosis, inhibiting synthesis of oleic acid and tuberculostearic acid.[1]
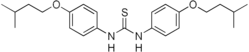 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.824 |
| Chemical and physical data | |
| Formula | C23H32N2O2S |
| Molar mass | 400.58 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Thiocarlide has considerable antimycobacterial activity in vitro and is effective against multi-drug resistant strains of Mycobacterium tuberculosis.[2] Isoxyl inhibits M. bovis with six hours of exposure, which is similar to isoniazid and ethionamide, two other prominent anti-TB drugs. Unlike these two drugs, however, isoxyl also partially inhibits the synthesis of fatty acids.
Thiocarlide was developed by a Belgian company, Continental Pharma S.A. Belgo-Canadienne in Brussels, Belgium. The head researcher was Professor N. P. Buu-Hoi, head of Continental Pharma's Research Division.
References
- Phetsuksiri B, Jackson M, Scherman H, et al. (December 2003). "Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis". J. Biol. Chem. 278 (52): 53123–30. doi:10.1074/jbc.M311209200. PMC 4747054. PMID 14559907.
- Phetsuksiri B, Baulard AR, Cooper AM, et al. (May 1999). "Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis". Antimicrob. Agents Chemother. 43 (5): 1042–51. doi:10.1128/AAC.43.5.1042. PMC 89109. PMID 10223912.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.