Carvacrol
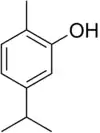
Carvacrol, or cymophenol, C6H3(CH3)(OH)C3H7, is a monoterpenoid phenol. It has a characteristic pungent, warm odor of oregano.[4]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methyl-5-(propan-2-yl)phenol[2] | |
| Systematic IUPAC name
2-Methyl-5-(propan-2-yl)benzenol | |
| Other names
Carvacrol 5-Isopropyl-2-methylphenol 2-Methyl-5-(1-methylethyl)phenol Isothymol Carvativir | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.173 |
IUPHAR/BPS |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H14O | |
| Molar mass | 150.217 g/mol |
| Density | 0.9772 g/cm3 at 20 °C |
| Melting point | 1 °C (34 °F; 274 K) |
| Boiling point | 237.7 °C (459.9 °F; 510.8 K) |
| insoluble | |
| Solubility | soluble in ethanol, diethyl ether, carbon tetrachloride, acetone[3] |
| −1.091×10−4 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Natural occurrence
Carvacrol is present in the essential oil of Origanum vulgare (oregano), oil of thyme, oil obtained from pepperwort, and wild bergamot.[5] The essential oil of thyme subspecies contains between 5% and 75% of carvacrol, while Satureja (savory) subspecies have a content between 1% and 45%.[6] Origanum majorana (marjoram) and Dittany of Crete are rich in carvacrol, 50% and 60–80% respectively.[7]
It is also found in tequila[8] and Lippia graveolens (Mexican oregano) in the verbena family.
Sources
- Monarda didyma[9]
- Nigella sativa[10]
- Origanum compactum[11]
- Origanum dictamnus[12]
- Origanum microphyllum[13]
- Origanum onites[14][15]
- Origanum scabrum[13]
- Origanum syriacum[16]
- Origanum vulgare[17][18]
- Plectranthus amboinicus
- Thymus glandulosus[11]
- Lavandula multifida
- Origanum minutiflorum
- Satureja thymbra
Synthesis and derivatives
Carvacrol may be synthetically prepared by a number of routes. The fusion of cymol sulfonic acid with caustic potash results in desulfonation. By the action of nitrous acid on 1-methyl-2-amino-4-propyl benzene, one effects diazotization. Prolonged heating of camphor and iodine or carvone with glacial phosphoric acid have also been demonstrated. The dehydrogenation of carvone with a palladium-carbon catalyst has been established.[5]
It has also been prepared by transalkylation of isopropylated cresols.[19]
It is extracted from Origanum oil by means of a 50% potash solution. It is a thick oil that sets at 20 °C to a mass of crystals of melting point 0 °C, and boiling point 236–237 °C. Oxidation with ferric chloride converts it into dicarvacrol, whilst phosphorus pentachloride transforms it into chlorcymol.[5]
Antimicrobial effects
In vitro, carvacrol has antimicrobial activity against 25 different phytopathogenic bacteria and strains including:[20] Cladosporium herbarum,[20] Penicillium glabrum,[20] and fungi such as Fusarium verticillioides/F. moniliforme, Rhizoctonia solani/R. solani, Sclerotinia sclerotiorum, Phytophthora capsici,[20] and Pseudomonas syringae.[21]
Compendial status
- British Pharmacopoeia[22]
See also
- Thymol
- Essential oil
Notes and references
- "Carvacrol data sheet from Sigma-Aldrich".
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 3–346. ISBN 978-0-8493-0594-8.
- Ultee, A.; Slump, R. A.; Steging, G.; Smid, E. J. (2000). "Antimicrobial activity of carvacrol toward Bacillus cereus on rice". Journal of Food Protection. 63 (5): 620–624. doi:10.4315/0362-028x-63.5.620. PMID 10826719.
- One or more of the preceding sentences incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "Carvacrol". Encyclopædia Britannica. Vol. 5 (11th ed.). Cambridge University Press. p. 437.
- Vladić, J.; Zeković, Z.; Jokić, S.; Svilović, S.; Kovačević, S.; Vidović, S. (November 2016). "Winter savory: Supercritical carbon dioxide extraction and mathematical modeling of extraction process". The Journal of Supercritical Fluids. 117: 89–97. doi:10.1016/j.supflu.2016.05.027.
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. (2004). "Constituents of aromatic plants: Carvacrol". Fitoterapia. 75 (7–8): 801–804. doi:10.1016/j.fitote.2004.05.002. PMID 15567271.
- De León Rodríguez, A.; Escalante Minakata, P.; Jiménez García, M. I.; Ordóñez Acevedo, L. G.; Flores Flores, J. L.; Barba de la Rosa, A. P. (2008). "Characterization of volatile compounds from ethnic Agave alcoholic beverages by gas chromatography-mass spectrometry". Food Technology and Biotechnology. 46 (4): 448–455.
- Mazza, G.; Kiehn, F. A.; Marshall, H. H. (1993). "Monarda: A source of geraniol, linalool, thymol and carvacrol-rich essential oils". In Janick, J.; Simon, J. E. (eds.). New Crops. New York: Wiley. pp. 628–631. ISBN 0-471-59374-5.
- Zawirska-Wojtasiak, R.; Mildner-Szkudlarz, S.; Wąsowicz, E.; Pacyński, M. (2010). "Gas chromatography, sensory analysis and electronic nose in the evaluation of black cumin (Nigella sativa L.) aroma quality" (PDF). Herba Polonica.
- Bouchra, C.; Achouri, M.; Idrissi Hassani, L. M.; Hmamouchi, M. (2003). "Chemical composition and antifungal activity of essential oils of seven Moroccan Labiatae against Botrytis cinerea Pers: Fr". Journal of Ethnopharmacology. 89 (1): 165–169. doi:10.1016/S0378-8741(03)00275-7. PMID 14522450.
- Liolios, C. C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. (2009). "Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity". Food Chemistry. 112 (1): 77–83. doi:10.1016/j.foodchem.2008.05.060.
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I. B. (2001). "Composition and antimicrobial activity of the essential oils of two Origanum species". Journal of Agricultural and Food Chemistry. 49 (9): 4168–4170. doi:10.1021/jf001494m. PMID 11559104.
- Coşkun, Ş.; Girişgin, O.; Kürkçüoğlu, M.; Malyer, H.; Girişgin, A. O.; Kırımer, N.; Başer, K. H. (2008). "Acaricidal efficacy of Origanum onites L. essential oil against Rhipicephalus turanicus (Ixodidae)". Parasitology Research. 103 (2): 259–261. doi:10.1007/s00436-008-0956-x. PMID 18438729.
- Ruberto, G.; Biondi, D.; Meli, R.; Piattelli, M. (1993). "Volatile flavour components of Sicilian Origanum onites L.". Flavour and Fragrance Journal. 8 (4): 197–200. doi:10.1002/ffj.2730080406.
- Ghasemi Pirbalouti, A.; Rahimmalek, M.; Malekpoor, F.; Karimi, A. (2011). "Variation in antibacterial activity, thymol and carvacrol contents of wild populations of Thymus daenensis subsp. daenensis Celak" (PDF). Plant Omics. 4: 209–214.
- Kanias, G. D.; Souleles, C.; Loukis, A.; Philotheou-Panou, E. (1998). "Trace elements and essential oil composition in chemotypes of the aromatic plant Origanum vulgare". Journal of Radioanalytical and Nuclear Chemistry. 227 (1–2): 23–31. doi:10.1007/BF02386426.
- Figiel, A.; Szumny, A.; Gutiérrez Ortiz, A.; Carbonell Barrachina, Á. A. (2010). "Composition of oregano essential oil (Origanum vulgare) as affected by drying method". Journal of Food Engineering. 98 (2): 240–247. doi:10.1016/j.jfoodeng.2010.01.002.
- Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef; Garbe (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- Andersen, A. (2006). "Final report on the safety assessment of sodium p-chloro-m-cresol, p-chloro-m-cresol, chlorothymol, mixed cresols, m-cresol, o-cresol, p-cresol, isopropyl cresols, thymol, o-cymen-5-ol, and carvacrol". International Journal of Toxicology. 25: 29–127. doi:10.1080/10915810600716653. PMID 16835130.
- Ni, Peien; Wang, Lei; Deng, Bohan; Jiu, Songtao; Ma, Chao; Zhang, Caixi; Almeida, Adelaide; Wang, Dapeng; Xu, Wenping; Wang, Shiping (2020-06-02). "Combined Application of Bacteriophages and Carvacrol in the Control of Pseudomonas syringae pv. actinidiae Planktonic and Biofilm Forms". Microorganisms. MDPI AG. 8 (6): 837. doi:10.3390/microorganisms8060837. ISSN 2076-2607. PMC 7356356.
- "Index" (PDF). British Pharmacopoeia. 2009. Archived from the original (PDF) on 11 April 2009. Retrieved 29 March 2010.