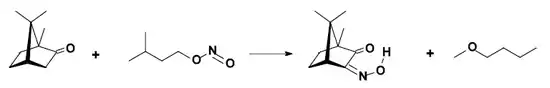Camphor
Camphor (/ˈkæmfər/) is a waxy, flammable, transparent solid with a strong aroma.[5] It is a terpenoid with the chemical formula C10H16O. It is found in the wood of the camphor laurel (Cinnamomum camphora), a large evergreen tree found in East Asia; and in the kapur tree (Dryobalanops sp.), a tall timber tree from South East Asia. It also occurs in some other related trees in the laurel family, notably Ocotea usambarensis. Rosemary leaves (Rosmarinus officinalis) contain 0.05 to 0.5% camphor,[6] while camphorweed (Heterotheca) contains some 5%.[7] A major source of camphor in Asia is camphor basil (the parent of African blue basil). Camphor can also be synthetically produced from oil of turpentine.
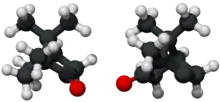
 (+)- and (−)-camphor | |
 | |
| Names | |
|---|---|
| IUPAC name
1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one | |
| Other names
2-Bornanone; Bornan-2-one; 2-Camphanone; Formosa | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
Beilstein Reference |
1907611 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.860 |
| EC Number |
|
Gmelin Reference |
83275 |
IUPHAR/BPS |
|
| KEGG | |
| MeSH | Camphor |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 2717 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H16O | |
| Molar mass | 152.237 g·mol−1 |
| Appearance | White, translucent crystals |
| Odor | Fragrant and penetrating |
| Density | 0.992 g·cm−3 |
| Melting point | 175–177 °C (347–351 °F; 448–450 K) |
| Boiling point | 209 °C (408 °F; 482 K) |
| 1.2 g·dm−3 | |
| Solubility in acetone | ~2500 g·dm−3 |
| Solubility in acetic acid | ~2000 g·dm−3 |
| Solubility in diethyl ether | ~2000 g·dm−3 |
| Solubility in chloroform | ~1000 g·dm−3 |
| Solubility in ethanol | ~1000 g·dm−3 |
| log P | 2.089 |
| Vapor pressure | 4 mmHg (at 70 °C) |
Chiral rotation ([α]D) |
+44.1° |
| −103×10−6 cm3/mol | |
| Pharmacology | |
| C01EB02 (WHO) | |
| Hazards | |
| GHS labelling: | |
   | |
| Warning | |
Hazard statements |
H228, H302, H332, H371 |
Precautionary statements |
P210, P240, P241, P260, P261, P264, P270, P271, P280, P301+P312, P304+P312, P304+P340, P309+P311, P312, P330, P370+P378, P405, P501 |
| NFPA 704 (fire diamond) | |
| Flash point | 54 °C (129 °F; 327 K) |
Autoignition temperature |
466 °C (871 °F; 739 K) |
| Explosive limits | 0.6–3.5%[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1310 mg/kg (oral, mouse)[4] |
LDLo (lowest published) |
800 mg/kg (dog, oral) 2000 mg/kg (rabbit, oral)[4] |
LCLo (lowest published) |
400 mg/m3 (mouse, 3 hr)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 2 mg/m3[3] |
REL (Recommended) |
TWA 2 mg/m3[3] |
IDLH (Immediate danger) |
200 mg/m3[3] |
| Related compounds | |
Related Ketones |
Fenchone, Thujone |
Related compounds |
Camphene, Pinene, Borneol, Isoborneol, Camphorsulfonic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
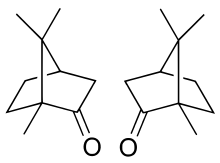
The molecule has two possible enantiomers as shown in the structural diagrams. The structure on the left is the naturally occurring (+)-camphor ((1R,4R)-bornan-2-one), while its mirror image shown on the right is the (−)-camphor ((1S,4S)-bornan-2-one).
Camphor is used for its scent, as an embalming fluid, as topical medication, as a manufacturing chemical, and in religious ceremonies.
Etymology
The word camphor derived in the 14th century from Old French: camphre, itself from Medieval Latin: camfora, from Arabic: كافور, romanized: kāfūr, perhaps through Sanskrit: कर्पुरम्, apparently from Austronesian Malay: kapur 'lime' (chalk).[8]
History
In Old Malay, camphor was called kapur barus, meaning "the chalk of Barus", referring to Barus, an ancient port near modern Sibolga on the western coast of Sumatra.[9] This port traded in camphor extracted from the Borneo camphor trees (Dryobalanops aromatica) that were abundant in the region.[10]
Production

Natural camphor
Camphor has been produced as a forest product for centuries, condensed from the vapor given off by the roasting of wood chips cut from the relevant trees, and later by passing steam through the pulverized wood and condensing the vapors.[11] By the early 19th century most camphor tree reserves had been depleted with the remaining large stands in Japan and Taiwan, with Taiwanese production greatly exceeding Japanese. Camphor was one of the primary resources extracted by Taiwan's colonial powers as well as one of the most lucrative. First the Chinese and then the Japanese established monopolies on Taiwanese camphor. In 1868, a British naval force sailed into Anping harbor and the local British representative demanded the end of the Chinese camphor monopoly. After the local imperial representative refused, the British bombarded the town and took the harbor. The "camphor regulations” negotiated between the two sides subsequently saw a brief end to the camphor monopoly.[12]
Artificial camphor
When its use in the nascent chemical industries (discussed below) greatly increased the volume of demand in the late 19th century, potential for changes in supply and in price followed. In 1911 Robert Kennedy Duncan, an industrial chemist and educator, related that the Imperial Japanese government had recently (1907–1908) tried to monopolize the production of natural camphor as a forest product in Asia but that the monopoly was prevented by the development of the total synthesis alternatives,[13] which began in "purely academic and wholly uncommercial"[13] form with Gustav Komppa's first report:
"... but it sealed the fate of the Japanese monopoly ... For no sooner was it accomplished than it excited the attention of a new army of investigators—the industrial chemists. The patent offices of the world were soon crowded with alleged commercial syntheses of camphor, and of the favored processes companies were formed to exploit them, factories resulted, and in the incredibly short time of two years after its academic synthesis artificial camphor, every whit as good as the natural product, entered the markets of the world ... And yet artificial camphor does not—and cannot—displace the natural product to an extent sufficient to ruin the camphor-growing industry. Its sole present and probable future function is to act as a permanent check to monopolization, to act as a balance-wheel to regulate prices within reasonable limits."[13]: 133–134
This ongoing check on price growth was confirmed in 1942 in a monograph on DuPont's history, where William S. Dutton said, "Indispensable in the manufacture of pyroxylin plastics, natural camphor imported from Formosa and selling normally for about 50 cents a pound, reached the high price of $3.75 in 1918 [amid the global trade disruption and high explosives demand that World War I created]. The organic chemists at DuPont replied by synthesizing camphor from the turpentine of Southern pine stumps, with the result that the price of industrial camphor sold in carload lots in 1939 was between 32 cents and 35 cents a pound."[14]: 293
Komppa's synthesis
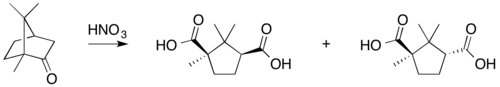
The background of Gustaf Komppa's synthesis was as follows. In the 19th century, it was known that nitric acid oxidizes camphor into camphoric acid. Haller and Blanc published a semisynthesis of camphor from camphoric acid. Although they demonstrated its structure, they were unable to prove it. The first complete total synthesis of camphoric acid was published by Komppa in 1903. Its inputs were diethyl oxalate and 3,3-dimethylpentanoic acid, which reacted by Claisen condensation to yield diketocamphoric acid. Methylation with methyl iodide and a complicated reduction procedure produced camphoric acid. William Perkin published another synthesis a short time later. Previously, some organic compounds (such as urea) had been synthesized in the laboratory as a proof of concept, but camphor was a scarce natural product with a worldwide demand. Komppa realized this, and began industrial production of camphor in Tainionkoski, Finland, in 1907 (with plenty of competition, as Kennedy Duncan reported).
Synthesis from alpha-pinene
Camphor can be produced from alpha-pinene, which is abundant in the oils of coniferous trees and can be distilled from turpentine produced as a side product of chemical pulping. With acetic acid as the solvent and with catalysis by a strong acid, alpha-pinene readily rearranges into camphene, which in turn undergoes Wagner–Meerwein rearrangement into the isobornyl cation, which is captured by acetate to give isobornyl acetate. Hydrolysis into isoborneol followed by oxidation gives racemic camphor. By contrast, camphor occurs naturally as D-camphor, the (R)-enantiomer.
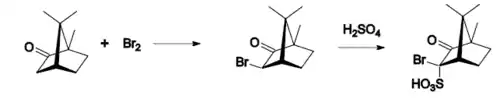
Reactions
Typical camphor reactions are:
- bromination,
- oxidation with nitric acid,
- conversion to isonitrosocamphor.
Camphor can also be reduced to isoborneol using sodium borohydride.
In 1998, K. Chakrabarti and coworkers from the Indian Association for the Cultivation of Science, Kolkata, prepared diamond thin film using camphor as the precursor for chemical vapor deposition.[15]
In 2007, carbon nanotubes were successfully synthesized using camphor in chemical vapor deposition process.[16]
Biochemistry
Biosynthesis
In biosynthesis, camphor is produced from geranyl pyrophosphate, via cyclisation of linaloyl pyrophosphate to bornyl pyrophosphate, followed by hydrolysis to borneol and oxidation to camphor.
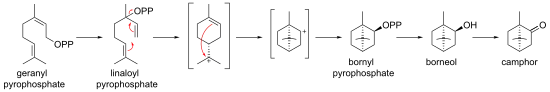 Biosynthesis of camphor from geranyl pyrophosphate
Biosynthesis of camphor from geranyl pyrophosphate
Alternative medicine

Camphor has been used as a folk medicine over centuries, probably most commonly as a decongestant.[17] Camphor was used in ancient Sumatra to treat sprains, swellings, and inflammation.[18] Camphor also was used for centuries in traditional Chinese medicine for various purposes.[17] In Europe, camphor was used after the Black Death era.[19]
In the 20th century, camphor was used as an analeptic by injection,[20] and to induce seizures in schizophrenic people in an attempt to treat psychosis.[21]
Topical medication
Camphor is commonly applied as a topical medication as a skin cream or ointment to relieve itching from insect bites, minor skin irritation, or joint pain.[22] It is absorbed in the skin epidermis,[22] where it stimulates nerve endings sensitive to heat and cold, producing a warm sensation when vigorously applied, or a cool sensation when applied gently, indicating its properties as a counterirritant.[17] The action on nerve endings also induces a slight local analgesia.[23]
Toxicity
Applied on skin, camphor may cause allergic reactions in some people; when ingested by mouth, camphor cream or ointment is poisonous.[22] In high ingested doses, camphor produces symptoms of irritability, disorientation, lethargy, muscle spasms, vomiting, abdominal cramps, convulsions, and seizures.[24] Lethal doses by ingestion in adults are in the range 50–500 mg/kg (orally). Generally, ingestion of two grams causes serious toxicity and four grams is potentially lethal.[25]
Respiratory aerosol
Camphor is also used via an aerosol, typically by steam inhalation, sometimes in the form of branded nasal inhaler sticks, to inhibit coughing and relieve upper airway congestion due to the common cold.[26] However, the clinical efficiency of these remedies is challenged.[27]
Camphor has limited use in veterinary medicine as a respiratory stimulant for horses.[28]
Physical uses
The sublimating capability of camphor gives it several uses. Since camphor is so flammable, one use camphor was put to was by marksmen to blacken the front and rear sights of rifles to prevent the sights from reflecting. Shiny sights are distracting. After the sights were cleaned, a marksmen would blacken them by holding the sight briefly in a small flame so a uniform coating of soot would accumulate. Many substances could provide this soot. But some marksmen "always blackened their sights by using a small piece of camphor."[29]
Plastics
The first significant manmade plastics were low-nitrogen (or "soluble") nitrocellulose (pyroxylin) plastics. In the early decades of the plastics industry, camphor was used in immense quantities[13]: 130 as the plasticizer that creates celluloid from nitrocellulose, in nitrocellulose lacquers and other plastics and lacquers.
Pest deterrent and preservative
Camphor is believed to be toxic to insects and is thus sometimes used as a repellent.[30] Camphor is used as an alternative to mothballs. Camphor crystals are sometimes used to prevent damage to insect collections by other small insects. It is kept in clothes used on special occasions and festivals, and also in cupboard corners as a cockroach repellent. The smoke of camphor crystal or camphor incense sticks can be used as an environmentally-friendly mosquito repellent.[31]
Recent studies have indicated that camphor essential oil can be used as an effective fumigant against red fire ants, as it affects the attacking, climbing, and feeding behavior of major and minor workers.[32]
Camphor is also used as an antimicrobial substance. In embalming, camphor oil was one of the ingredients used by ancient Egyptians for mummification.[33]
Solid camphor releases fumes that form a rust-preventative coating and is therefore stored in tool chests to protect tools against rust.[34]
Culinary uses
One of the earliest known recipes for ice cream dating to the Tang dynasty includes camphor as an ingredient.[37] It was used to flavor leavened bread in ancient Egypt.[38] In ancient and medieval Europe, camphor was used as an ingredient in sweets. It was used in a wide variety of both savory and sweet dishes in medieval Arabic language cookbooks, such as al-Kitab al-Ṭabikh compiled by ibn Sayyār al-Warrāq in the 10th century.[39] It also was used in sweet and savory dishes in the Ni'matnama, according to a book written in the late 15th century for the sultans of Mandu.[40]
Religion
Hindu religious ceremonies
Camphor is widely used in Hindu religious ceremonies. It is put on a stand called karpur dāni in India. Aarti is performed after setting fire to it usually as the last step of puja.[41]
See also
- 1,4-Dichlorobenzene
- Citral
- Eucalyptol
- Lavender
- Vaporizer
References
- The Merck Index, 7th edition, Merck & Co., Rahway, New Jersey, 1960
- Handbook of Chemistry and Physics, CRC Press, Ann Arbor, Michigan, USA
- NIOSH Pocket Guide to Chemical Hazards. "#0096". National Institute for Occupational Safety and Health (NIOSH).
- "Camphor (synthetic)". National Institute for Occupational Safety and Health (NIOSH). 4 December 2014. Archived from the original on 13 March 2015. Retrieved 19 February 2015.
- Mann JC, Hobbs JB, Banthorpe DV, Harborne JB (1994). Natural products: their chemistry and biological significance. Harlow, Essex, England: Longman Scientific & Technical. pp. 309–11. ISBN 978-0-582-06009-8.
- "Rosemary". Drugs.com. Archived from the original on 14 September 2016. Retrieved 23 July 2016.
- Lincoln, D.E.; Lawrence, B.M. (1984). "The volatile constituents of camphorweed, Heterotheca subaxillaris". Phytochemistry. 23 (4): 933–934. doi:10.1016/S0031-9422(00)85073-6.
- "Camphor". Online Etymology Dictionary. Douglas Harper. Archived from the original on 23 May 2021. Retrieved 23 May 2021.
- Drakard, Jane (1989). "An Indian Ocean Port: Sources for the Earlier History of Barus". Archipel. 37: 53–82. doi:10.3406/arch.1989.2562.
- Laufer, Berthold (1919). "SINO-IRANICA: Chinese Contributions to the History of Civilization in Ancient Iran". Publications of the Field Museum of Natural History. Anthropological Series. 15 (3): 478–479. ISSN 0894-8380. JSTOR 29782155. Archived from the original on 2020-11-17. Retrieved 2022-01-22.
- "Camphor". britannica.com. Archived from the original on December 13, 2018. Retrieved December 12, 2018.
- Cheung, Han. "Taiwan in Time: The camphor dispute". www.taipeitimes.com. Taipei Times. Archived from the original on 15 November 2020. Retrieved 14 November 2020.
- Kennedy Duncan, Robert (1911), "Camphor: An Industry Revolutionized", Some Chemical Problems of Today, Harper and Brothers, LCCN 11026192, archived from the original on 2020-07-27, retrieved 2018-01-17.
- Dutton, William S. (1942), Du Pont: One Hundred and Forty Years, Charles Scribner's Sons, LCCN 42011897, archived from the original on 2020-08-04, retrieved 2018-01-17.
- Chakrabarti K, Chakrabarti R, Chattopadhyay KK, Chaudhuri S, Pal AK (1998). "Nano-diamond films produced from CVD of camphor". Diam Relat Mater. 7 (6): 845–52. Bibcode:1998DRM.....7..845C. doi:10.1016/S0925-9635(97)00312-9.
- Kumar M, Ando Y (2007). "Carbon Nanotubes from Camphor: An Environment-Friendly Nanotechnology". J Phys Conf Ser. 61 (1): 643–6. Bibcode:2007JPhCS..61..643K. doi:10.1088/1742-6596/61/1/129.
- Goodman and Gilman, Pharmacological Basis of Therapeutics, Macmillan 1965, p. 982-983. ISBN 9780071468046.
- Miller, Charles. History of Sumatra : An account of Sumatra. p. 121.
- Chen, Weiyang; Vermaak, Ilze; Viljoen, Alvaro (2013-05-10). "Camphor--a fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon--a review". Molecules (Basel, Switzerland). 18 (5): 5434–5454. doi:10.3390/molecules18055434. ISSN 1420-3049. PMC 6270224. PMID 23666009.
- Wax, P. M. (1997). "Analeptic use in clinical toxicology: a historical appraisal". Journal of Toxicology. Clinical Toxicology. 35 (2): 203–209. doi:10.3109/15563659709001195. ISSN 0731-3810. PMID 9120893.
- Bangen, Hans: Geschichte der medikamentösen Therapie der Schizophrenie. Berlin 1992, Page 51-55 ISBN 3-927408-82-4
- "Camphor cream and ointment". Drugs.com. August 25, 2019. Archived from the original on 3 May 2021. Retrieved 19 February 2020.
- Bonica's Management of Pain (4th ed.). Philadelphia, Baltimore: Wolters Kluwer - Lippincott Williams & Wilkins. 2009. p. 29. ISBN 9780781768276.
- "Camphor overdose". MedlinePlus, National Library of Medicine, US National Institutes of Health. January 12, 2019. Archived from the original on July 5, 2016. Retrieved February 19, 2020.
- "Poisons Information Monograph: Camphor". International Programme on Chemical Safety. Archived from the original on 2021-02-11. Retrieved 2008-12-12.
- "Camphor Inhalation Liquid: Indications, Side Effects, Warnings". Drugs.com. Retrieved 2022-10-28.
- Burrow, A.; Eccles, R; Jones, AS (July 1983). "The effects of camphor, eucalyptus and menthol vapour on nasal resistance to airflow and nasal sensation". Acta Oto-Laryngologica. 96 (1–2): 157–161. doi:10.3109/00016488309132886. ISSN 0001-6489. PMID 6613544.
- "Camphor injection (Canada)". Drugs.com. February 6, 2020. Archived from the original on 27 July 2020. Retrieved 19 February 2020.
- Chapel, Charles Edward, "The Boy's Book of Rifles, " Coward-McCann, Inc., New York, Copyright 1948, page 96.
- The Housekeeper's Almanac, or, the Young Wife's Oracle! for 1840!. No. 134. New-York: Elton, 1840. Print.
- Ghosh, G.K. (2000). Biopesticide and Integrated Pest Management. APH Publishing. ISBN 978-8-176-48135-9.
- Fu JT, Tang L, Li WS, Wang K, Cheng DM, Zhang ZX (2015). "Carbon Nanotubes from Camphor: An Environment-Friendly Nanotechnology". J Insect Sci. 15 (1): 129. doi:10.1093/jisesa/iev112. PMC 4664941. PMID 26392574.
- "Mummy-making complexity revealed". Archived from the original on 18 May 2015. Retrieved 3 July 2018.
- "Remove rust from tools". www.naturalhandyman.com. Retrieved 2022-04-28.
- Groom, N. (2012). The Perfume Handbook. Springer Netherlands. ISBN 978-9-401-12296-2.
- Donkin, R. A. (1999). Dragon's Brain Perfume: An Historical Geography of Camphor. Brill. ISBN 978-9-004-10983-4.
- Clarke, Chris (2004). Science of Ice Cream. Royal Society of chemistry. p. 4.
- Muller, H. G. (1986). Baking and bakeries. Shire Publications. p. 7. ISBN 9780852638019.
- Nasrallah, Nawal (2007). Annals of the Caliphs' Kitchens: Ibn Sayyâr al-Warrâq's Tenth-century Baghdadi Cookbook. Islamic History and Civilization, 70. Leiden, The Netherlands: Brill. ISBN 978-0-415-35059-4.
- Titley, Norah M. (2004). The Ni'matnama Manuscript of the Sultans of Mandu: The Sultan's Book of Delights. Routledge Studies in South Asia. London, UK: Routledge. ISBN 978-0-415-35059-4.
- Bahadur, Om Lata (1996). The book of Hindu festivals and ceremonies (3rd ed.). New Delhi: UBS Publishers Distributors ltd. pp. 172–3. ISBN 978-81-86112-23-6.
- Quran 76:5 pg 578
- Levi Cooper, "Do we need to fast during a pandemic? Archived 2022-01-09 at the Wayback Machine" The Jerusalem Post, July 9, 2020
External links
- INCHEM at IPCS (International Programme on Chemical Safety)
- NIOSH Pocket Guide to Chemical Hazards - Camphor at Centers for Disease Control and Prevention