Acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula (CH3)2CO.[18] It is the simplest and smallest ketone (>C=O). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
| |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| IUPAC name
Acetone[2] | |||
| Preferred IUPAC name
Propan-2-one[3] | |||
| Systematic IUPAC name
2-Propanone[4] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 3DMet | |||
Beilstein Reference |
635680 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.602 | ||
| EC Number |
| ||
Gmelin Reference |
1466 | ||
| KEGG | |||
| MeSH | Acetone | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1090 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H6O | |||
| Molar mass | 58.080 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Pungent, irritating, floral, cucumber-like | ||
| Density | 0.7845 g/cm3 (25 °C) | ||
| Melting point | −94.7 °C (−138.5 °F; 178.5 K)[10] | ||
| Boiling point | 56.05 °C (132.89 °F; 329.20 K)[10] | ||
| Miscible | |||
| Solubility | Miscible in benzene, diethyl ether, methanol, chloroform, ethanol[11] | ||
| log P | −0.16[12] | ||
| Vapor pressure |
| ||
| Acidity (pKa) | |||
| −33.78·10−6 cm3/mol | |||
Refractive index (nD) |
1.3588 (VD = 54.46) | ||
| Viscosity | 0.295 mPa·s (25 °C)[11] | ||
| Structure | |||
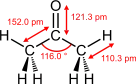
| Trigonal planar at C2 | |||
Molecular shape |
Dihedral at C2 | ||
Dipole moment |
2.91 D | ||
| Thermochemistry | |||
Heat capacity (C) |
125.45 J/(mol·K) | ||
Std molar entropy (S⦵298) |
200.4 J/(mol·K) | ||
Std enthalpy of formation (ΔfH⦵298) |
(−250.03) – (−248.77) kJ/mol | ||
Std enthalpy of combustion (ΔcH⦵298) |
−1.772 MJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Mildly toxic[15] | ||
| GHS labelling: | |||
  | |||
| Danger | |||
Hazard statements |
H225, H302, H319, H336, H373 | ||
Precautionary statements |
P210, P235, P260, P305+P351+P338 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | −20 °C (−4 °F; 253 K) | ||
Autoignition temperature |
465 °C (869 °F; 738 K) | ||
| Explosive limits | 2.6–12.8%[16] | ||
Threshold limit value (TLV) |
1185 mg/m3 (TWA), 2375 mg/m3 (STEL) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
| ||
LC50 (median concentration) |
20,702 ppm (rat, 8 h)[17] | ||
LCLo (lowest published) |
45,455 ppm (mouse, 1 h)[17] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
1000 ppm (2400 mg/m3)[6] | ||
REL (Recommended) |
TWA 250 ppm (590 mg/m3)[6] | ||
IDLH (Immediate danger) |
2500 ppm[6] | ||
| Related compounds | |||
Related compounds |
|||
| Supplementary data page | |||
| Acetone (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Acetone is miscible with water and serves as an important organic solvent in its own right, in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and production of methyl methacrylate (and from that PMMA) as well as bisphenol A.[19][20] It is a common building block in organic chemistry. Familiar household uses of acetone are as the active ingredient in nail polish remover and as paint thinner. It has volatile organic compound (VOC) exempt status in the United States.[21]
Acetone is produced and disposed of in the human body through normal metabolic processes. It is normally present in blood and urine. People with diabetic ketoacidosis produce it in larger amounts. Reproductive toxicity tests show that it has low potential to cause reproductive problems. Ketogenic diets that increase ketone bodies (acetone, β-hydroxybutyric acid and acetoacetic acid) in the blood are used to counter epileptic attacks in infants and children who suffer from refractory epilepsy.[22]
History
Acetone was first produced by Andreas Libavius in 1606 by distillation of Lead(II) acetate.[23] [24]
In 1832, French chemist Jean-Baptiste Dumas and German chemist Justus von Liebig determined the empirical formula for acetone.[25][26] In 1833, the French chemist Antoine Bussy named acetone by adding the suffix -one to the stem of the corresponding acid (viz, acetic acid).[27] By 1852, English chemist Alexander William Williamson realized that acetone was methyl acetyl;[28] the following year, the French chemist Charles Frédéric Gerhardt concurred.[29] In 1865, the German chemist August Kekulé published the modern structural formula for acetone.[30][31] Johann Josef Loschmidt had presented the structure of acetone in 1861,[32] but his privately published booklet received little attention. During World War I, Chaim Weizmann developed the process for industrial production of acetone (Weizmann Process).[33]
Production
In 2010, the worldwide production capacity for acetone was estimated at 6.7 million tonnes per year.[34] With 1.56 million tonnes per year, the United States had the highest production capacity,[35] followed by Taiwan and mainland China. The largest producer of acetone is INEOS Phenol, owning 17% of the world's capacity, with also significant capacity (7–8%) by Mitsui, Sunoco and Shell in 2010.[34] INEOS Phenol also owns the world's largest production site (420,000 tonnes/annum) in Beveren (Belgium). Spot price of acetone in summer 2011 was 1100–1250 USD/tonne in the United States.[36]
Current method
Acetone is produced directly or indirectly from propylene. Approximately 83% of acetone is produced via the cumene process;[20] as a result, acetone production is tied to phenol production. In the cumene process, benzene is alkylated with propylene to produce cumene, which is oxidized by air to produce phenol and acetone:
Other processes involve the direct oxidation of propylene (Wacker-Hoechst process), or the hydration of propylene to give 2-propanol, which is oxidized (dehydrogenated) to acetone.[20]
Older methods
Previously, acetone was produced by the dry distillation of acetates, for example calcium acetate in ketonic decarboxylation.
After that time, during World War I, acetone was produced using acetone-butanol-ethanol fermentation with Clostridium acetobutylicum bacteria, which was developed by Chaim Weizmann (later the first president of Israel) in order to help the British war effort,[20] in the preparation of Cordite.[37] This acetone-butanol-ethanol fermentation was eventually abandoned when newer methods with better yields were found.[20]
Chemical properties
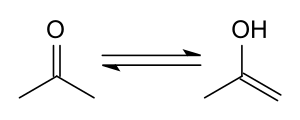
Keto/enol tautomerism
Like most ketones, acetone exhibits the keto–enol tautomerism in which the nominal keto structure (CH3)2C=O of acetone itself is in equilibrium with the enol isomer (CH3)C(OH)=(CH2) (prop-1-en-2-ol). In acetone vapor at ambient temperature, only 2.4×10−7% of the molecules are in the enol form.[38] Yet the enol form is chemically important in some chemical reactions.
Aldol condensation
In the presence of suitable catalysts, two acetone molecules also combine to form the compound diacetone alcohol (CH3)C=O(CH2)C(OH)(CH3)2, which on dehydration gives mesityl oxide (CH3)C=O(CH)=C(CH3)2. This product can further combine with another acetone molecule, with loss of another molecule of water, yielding phorone and other compounds.
Polymerisation
One might expect acetone to also form polymers and (possibly cyclic) oligomers of two types. In one type, units could be acetone molecules linked by ether bridges −O− derived by from the opening of the double bond, to give a polyketal-like (PKA) chain [−O−C(CH3)2−]n. The other type could be obtained through repeated aldol condensation, with one molecule of water removed at each step, yielding a poly(methylacetylene) (PMA) chain [−CH=C(CH3)−]n.[39]
PKA type
The conversion of acetone to a polyketal (PKA) would be analogous to the formation of paraformaldehyde from formol, and of trithioacetone from thioacetone. In 1960, Kargin, Kabanov and others observed that the thermodynamics of this process is unfavourable for liquid acetone, so that it (unlike thioacetone and formol) is not expected to polymerise spontaneously, even with catalysts. However, they observed that the thermodynamics became favourable for crystalline solid acetone at the melting point (−96 °C). They claimed to have obtained such a polymer (a white elastic solid, soluble in acetone, stable for several hours at room temperature) by depositing vapor of acetone, with some magnesium as a catalyst, onto a very cold surface.[40]
In 1962, Wasaburo Kawai reported the synthesis of a similar product, from liquid acetone cooled to −70 to −78 °C, using n-butyl lithium or triethylaluminium as catalysts. He claimed that the infrared absorption spectrum showed the presence of −O− linkages but no C=O groups.[41] However, conflicting results were obtained later by other investigators.[39]
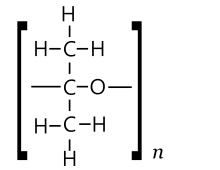
PMA type
The PMA type polymers of acetone would be equivalent to the product of polymerisation of propyne, except for a keto end group.[39]
Biochemistry
Biosynthesis
Small amounts of acetone are produced in the body by the decarboxylation of ketone bodies. Certain dietary patterns, including prolonged fasting and high-fat low-carbohydrate dieting, can produce ketosis, in which acetone is formed in body tissue. Certain health conditions, such as alcoholism and diabetes, can produce ketoacidosis, uncontrollable ketosis that leads to a sharp, and potentially fatal, increase in the acidity of the blood. Since it is a byproduct of fermentation, acetone is a byproduct of the distillery industry.
Acetone can be produced from the oxidation of ingested isopropanol, or from the spontaneous/enzymatic breakdown of acetoacetate (a ketone body) in ketotic individuals.
Metabolism
Although some biochemistry textbooks and current research publications[42] indicate that acetone cannot be metabolized, there is evidence to the contrary. It can then be metabolized either by CYP2E1 via methylglyoxal to D-lactate and pyruvate, and ultimately glucose/energy, or by a different pathway via propylene glycol to pyruvate, lactate, acetate (usable for energy) and propionaldehyde.[43][44][45]
Uses
Industrial
About a third of the world's acetone is used as a solvent, and a quarter is consumed as acetone cyanohydrin, a precursor to methyl methacrylate.[19]
Solvent
Acetone is a good solvent for many plastics and some synthetic fibers. It is used for thinning polyester resin, cleaning tools used with it, and dissolving two-part epoxies and superglue before they harden. It is used as one of the volatile components of some paints and varnishes. As a heavy-duty degreaser, it is useful in the preparation of metal prior to painting or soldering, and to remove rosin flux after soldering (to prevent adhesion of dirt and electrical leakage and perhaps corrosion or for cosmetic reasons), although it attacks many electronic components (for example polystyrene capacitors) so it is unsuitable for cleaning many circuit boards.
Acetylene carrier
Although itself flammable, acetone is used extensively as a solvent for the safe transportation and storage of acetylene, which cannot be safely pressurized as a pure compound. Vessels containing a porous material are first filled with acetone followed by acetylene, which dissolves into the acetone. One litre of acetone can dissolve around 250 litres of acetylene at a pressure of 10 bars (1.0 MPa).[46][47]
Chemical intermediate
Acetone is used to synthesize methyl methacrylate. It begins with the initial conversion of acetone to acetone cyanohydrin via reaction with hydrogen cyanide (HCN):
In a subsequent step, the nitrile is hydrolyzed to the unsaturated amide, which is esterified:
The third major use of acetone (about 20%)[19] is synthesizing bisphenol A. Bisphenol A is a component of many polymers such as polycarbonates, polyurethanes, and epoxy resins. The synthesis involves the condensation of acetone with phenol:
Many millions of kilograms of acetone are consumed in the production of the solvents methyl isobutyl alcohol and methyl isobutyl ketone. These products arise via an initial aldol condensation to give diacetone alcohol.[20]
Condensation with acetylene gives 2-methylbut-3-yn-2-ol, precursor to synthetic terpenes and terpenoids.
Chromatography

Spectroscopy techniques are useful when the sample being tested is pure, or a very common mixture. When an unknown mixture is being analyzed it must be broken down into its individual parts. Chromatography techniques can be used to break apart mixtures into their components allowing for each part to be analyzed separately.
Thin layer chromatography (TLC) is a quick alternative to more complex chromatography methods. TLC can be used to analyze inks and dyes by extracting the individual components.[48] This can be used to investigate notes or fibers left at the scene since each company's product is slightly different and those differences can be seen with TLC. The only limiting factor with TLC analysis is the necessity for the components to be soluble in whatever solution is used to carry the components up the analysis plate.[48] This solution is called the mobile phase.[48] The forensic chemist can compare unknowns with known standards by looking at the distance each component travelled.[48] This distance, when compared to the starting point, is known as the retention factor (Rf) for each extracted component.[48] If each Rf value matches a known sample, that is an indication of the unknown's identity.[48]
High-performance liquid chromatography can be used to extract individual components from a mixture dissolved in a solution. HPLC is used for nonvolatile mixtures that would not be suitable for gas chromatography.[49] This is useful in drug analysis where the pharmaceutical is a combination drug since the components would separate, or elute, at different times allowing for the verification of each component.[50] The eluates from the HPLC column are then fed into various detectors that produce a peak on a graph relative to its concentration as it elutes off the column. The most common type of detector is an ultraviolet-visible spectrometer as the most common item of interest tested with HPLC, pharmaceuticals, have UV absorbance.[51]
Gas chromatography (GC) performs the same function as liquid chromatography, but it is used for volatile mixtures. In forensic chemistry, the most common GC instruments use mass spectrometry as their detector.[52] GC-MS can be used in investigations of arson, poisoning, and explosions to determine exactly what was used. In theory, GC-MS instruments can detect substances whose concentrations are in the femtogram (10−15) range.[53] However, in practice, due to signal-to-noise ratios and other limiting factors, such as the age of the individual parts of the instrument, the practical detection limit for GC-MS is in the picogram (10−12) range.[54] GC-MS is also capable of quantifying the substances it detects; chemists can use this information to determine the effect the substance would have on an individual. GC-MS instruments need around 1,000 times more of the substance to quantify the amount than they need simply to detect it; the limit of quantification is typically in the nanogram (10−9) range.[54]
Chemical research
In the laboratory, acetone is used as a polar, aprotic solvent in a variety of organic reactions, such as SN2 reactions. The use of acetone solvent is critical for the Jones oxidation. It does not form an azeotrope with water (see azeotrope tables).[55] It is a common solvent for rinsing laboratory glassware because of its low cost and volatility. Despite its common use as a supposed drying agent, it is not effective except by bulk displacement and dilution. Acetone can be cooled with dry ice to −78 °C without freezing; acetone/dry ice baths are commonly used to conduct reactions at low temperatures. Acetone is fluorescent under ultraviolet light, and its vapor can be used as a fluorescent tracer in fluid flow experiments.[56]
Acetone is used to precipitate proteins.[57] Alternatives for protein precipitation are trichloroacetic acid or ethanol.
Cleaning
Low-grade acetone is also commonly used in academic laboratory settings as a glassware rinsing agent for removing residue and solids before a final wash.[58] Acetone leaves a small amount of residue on a surface when dried that is harmful to surface samples.
Low-temperature bath
A mixture of acetone and dry ice is a popular cooling bath that maintains a temperature of −78 °C as long as there is some dry ice left.[59]
Histology
Acetone is used in the field of pathology to find lymph nodes in fatty tissues for tumor staging (such as looking for lymph nodes in the fat surrounding the intestines).[60] This helps dissolve the fat, and hardens the nodes, making finding them easier.[61]
Acetone also used for destaining microscope slides of certain stains.[62]
Lewis base properties
Acetone is a weak Lewis base that forms adducts with soft acids like I2 and hard acids like phenol. Acetone also forms complexes with divalent metals.[63][64]
Drug solvent and excipient
Acetone is used as a solvent by the pharmaceutical industry and as a denaturant in denatured alcohol.[65] Acetone is also present as an excipient in some pharmaceutical drugs.[66]
Skin defatting
Dermatologists use acetone with alcohol for acne treatments to chemically peel dry skin. Common agents used today for chemical peeling are salicylic acid, glycolic acid, azelaic acid, 30% salicylic acid in ethanol, and trichloroacetic acid (TCA). Prior to chemexfoliation, the skin is cleaned and excess fat removed in a process called defatting. Acetone, Septisol, or a combination of these agents is commonly used in this process.
Anticonvulsant
Acetone has been shown to have anticonvulsant effects in animal models of epilepsy, in the absence of toxicity, when administered in millimolar concentrations.[67] It has been hypothesized that the high-fat low-carbohydrate ketogenic diet used clinically to control drug-resistant epilepsy in children works by elevating acetone in the brain.[67] Because of their higher energy requirements, children have higher acetone production than most adults – and the younger the child, the higher the expected production. This indicates that children are not uniquely susceptible to acetone exposure. External exposures are small compared to the exposures associated with the ketogenic diet.[68]
Domestic and other niche uses
Acetone is often the primary component in cleaning agents such as nail polish and superglue removers. It will attack some plastics, however.
Make-up artists use acetone to remove skin adhesive from the netting of wigs and mustaches by immersing the item in an acetone bath, then removing the softened glue residue with a stiff brush.
Acetone is often used for vapor polishing of printing artifacts on 3D-printed models printed with ABS plastic. The technique, called acetone vapor bath smoothing, involves placing the printed part in a sealed chamber containing a small amount of acetone, and heating to around 80 degrees Celsius for 10 minutes. This creates a vapor of acetone in the container. The acetone condenses evenly all over the part, causing the surface to soften and liquefy. Surface tension then smooths the semi-liquid plastic. When the part is removed from the chamber, the acetone component evaporates leaving a glassy-smooth part free of striation, patterning, and visible layer edges, common features in untreated 3D printed parts.[69]
Acetone efficiently removes felt-tipped pen marks from glass and metals.
Safety
Flammability
The most hazardous property of acetone is its extreme flammability. In small amounts Acetone burns with a dull blue flame, in larger amounts the evaporation of fuel causes incomplete combustion and a bright yellow flame. At temperatures greater than acetone's flash point of −20 °C (−4 °F), air mixtures of between 2.5% and 12.8% acetone, by volume, may explode or cause a flash fire. Vapors can flow along surfaces to distant ignition sources and flash back. Static discharge may also ignite acetone vapors, though acetone has a very high ignition initiation energy point and therefore accidental ignition is rare. Even pouring or spraying acetone over red-glowing coal will not ignite it, due to the high concentration of vapour and the cooling effect of evaporation of the liquid.[70] It auto-ignites at 465 °C (869 °F). Auto-ignition temperature is also dependent upon the exposure time, thus at some tests it is quoted as 525 °C. Also, industrial acetone is likely to contain a small amount of water which also inhibits ignition.
Acetone peroxide
When oxidized, acetone forms acetone peroxide as a byproduct, which is a highly unstable, primary high explosive compound. It may be formed accidentally, e.g. when waste hydrogen peroxide is poured into waste solvent containing acetone. Due to its instability, it is rarely used, despite its simple chemical synthesis.
Toxicity
Acetone has been studied extensively and is believed to exhibit only slight toxicity in normal use. There is no strong evidence of chronic health effects if basic precautions are followed.[71] It is generally recognized to have low acute and chronic toxicity if ingested and/or inhaled.[72] Acetone is not currently regarded as a carcinogen, a mutagenic chemical nor a concern for chronic neurotoxicity effects.[70]
Acetone can be found as an ingredient in a variety of consumer products ranging from cosmetics to processed and unprocessed foods. Acetone has been rated as a generally recognized as safe (GRAS) substance when present in beverages, baked foods, desserts, and preserves at concentrations ranging from 5 to 8 mg/L.[72]
Acetone is however an irritant, causing mild skin irritation and moderate to severe eye irritation. At high vapor concentrations, it may depress the central nervous system like many other solvents.[73] Acute toxicity for mice by ingestion (LD50) is 3 g/kg, and by inhalation (LC50) is 44 g/m3 over 4 hours.[74]
EPA Classification
In 1995, the United States Environmental Protection Agency (EPA) removed acetone from the list of "toxic chemicals" maintained under Section 313 of the Emergency Planning and Community Right to Know Act (EPCRA). In making that decision, EPA conducted an extensive review of the available toxicity data on acetone and found that acetone "exhibits acute toxicity only at levels that greatly exceed releases and resultant exposures", and further that acetone "exhibits low toxicity in chronic studies".
- Genotoxicity. Acetone has been tested in more than two dozen in vitro and in vivo assays. These studies indicate that acetone is not genotoxic.
- Carcinogenicity. EPA in 1995 concluded, "There is currently no evidence to suggest a concern for carcinogenicity". (EPCRA Review, described in Section 3.3). NTP scientists have recommended against chronic toxicity/carcinogenicity testing of acetone because "the prechronic studies only demonstrated a very mild toxic response at very high doses in rodents".
- Neurotoxicity and Developmental Neurotoxicity. The neurotoxic potential of both acetone and isopropanol, the metabolic precursor of acetone, have been extensively studied. These studies demonstrate that although exposure to high doses of acetone may cause transient central nervous system effects, acetone is not a neurotoxicant. A guideline developmental neurotoxicity study has been conducted with isopropanol, and no developmental neurotoxic effects were identified, even at the highest dose tested. (SIAR, pp. 1, 25, 31).
- Environmental. When the EPA exempted acetone from regulation as a volatile organic compound (VOC) in 1995, EPA stated that this exemption would "contribute to the achievement of several important environmental goals and would support EPA's pollution prevention efforts". 60 Fed. Reg. 31,634 (June 16, 1995). 60 Fed. Reg. 31,634 (June 16, 1995). EPA noted that acetone could be used as a substitute for several compounds that are listed as hazardous air pollutants (HAP) under section 112 of the Clean Air Act.
Environmental effects
Although acetone occurs naturally in the environment in plants, trees, volcanic gases, forest fires, and as a product of the breakdown of body fat,[75] the majority of the acetone released into the environment is of industrial origin. Acetone evaporates rapidly, even from water and soil. Once in the atmosphere, it has a 22-day half-life and is degraded by UV light via photolysis (primarily into methane and ethane.[76]) Consumption by microorganisms contributes to the dissipation of acetone in soil, animals, or waterways.[75]
The LD50 of acetone for fish is 8.3 g/L of water (or about 1%) over 96 hours, and its environmental half-life in water is about 1 to 10 days. Acetone may pose a significant risk of oxygen depletion in aquatic systems due to microbial consumption.[77]
Extraterrestrial occurrence
On 30 July 2015, scientists reported that upon the first touchdown of the Philae lander on comet 67P's surface, measurements by the COSAC and Ptolemy instruments revealed sixteen organic compounds, four of which were seen for the first time on a comet, including acetamide, acetone, methyl isocyanate, and propionaldehyde.[78][79][80]
References
- The Merck Index, 15th Ed. (2013), p. 13, Acetone Monograph 65, O'Neil: The Royal Society of Chemistry.(subscription required)
- ChemSpider lists 'acetone' as a valid, expert-verified name for what would systematically be called 'propan-2-one'.
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 723. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Acetone: IUPAC name
- Acetone in Linstrom, Peter J.; Mallard, William G. (eds.); NIST Chemistry WebBook, NIST Standard Reference Database Number 69, National Institute of Standards and Technology, Gaithersburg (MD)
- NIOSH Pocket Guide to Chemical Hazards. "#0004". National Institute for Occupational Safety and Health (NIOSH).
- Klamt, Andreas (2005). COSMO-RS: From Quantum Chemistry to Fluid Phase Thermodynamics and Drug Design. Elsevier. pp. 92–94. ISBN 978-0-444-51994-8.
- Ketones nomenclature: acetone
- Myers, Richard L. (2007). The 100 Most Important Chemical Compounds: A Reference Guide. Greenwood. pp. 4–6. ISBN 978-0-313-08057-9.
- Lide, David R. (ed) (2003). CRC Handbook of Chemistry and Physics, 84th Edition. CRC Press. Boca Raton, Florida; Section 3, Physical Constants of Organic Compounds.
- Properties of substance: acetone. chemister.ru.
- "acetone". ChemSrc. Retrieved 2018-04-13.
- Chiang, Yvonne; Kresge, A. Jerry; Tang, Yui S.; Wirz, Jakob (1984). "The pKa and keto-enol equilibrium constant of acetone in aqueous solution". Journal of the American Chemical Society. 106 (2): 460–462. doi:10.1021/ja00314a055.
- Bordwell, Frederick G. (1988). "Equilibrium acidities in dimethyl sulfoxide solution". Accounts of Chemical Research. 21 (12): 456–463. doi:10.1021/ar00156a004.
- Acetone toxicity
- "Working with modern hydrocarbon and oxygenated solvents: a guide to flammability". American Chemistry Council Solvents Industry Group. January 2008. p. 7. Archived from the original on 2009-06-01.
- "Acetone". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Allen, P.W.; Bowen, H.J.M.; Sutton, L.E.; Bastiansen, O. (1952). "The molecular structure of acetone". Transactions of the Faraday Society. 48: 991. doi:10.1039/TF9524800991.
- Acetone, World Petrochemicals report, January 2010
- Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.
- "Update: U.S. EPA Exempt Volatile Organic Compounds". American Coatings Association. 2018-01-30. Archived from the original on 2021-02-08. Retrieved 2019-03-20.
- Freeman, JM; Kossoff, EH; Hartman, AL (Mar 2007). "The ketogenic diet: one decade later". Pediatrics. 119 (3): 535–43. doi:10.1542/peds.2006-2447. PMID 17332207. S2CID 26629499.
- Libavius, Andreas (1606). Alchymia (in Latin). Frankfurt, (Germany): printed by Joannes Saurius, at the expense of Peter Kopff. p. 123. From p. 123: "QUINTA ESSENTIA PLUMBI. Calcem Saturni macera in aceto stillatitio per biduum in cineribus, & solvetur in acetum tenuis substantia, effunde, reponeque aliud, & sic perge, quoad tota subtilitas est extracta. Coagula acetum collectum in salem. Huic affunde spiritum vini circulatum aut alcalisatum. Circula per dies aliquot, destilla ut essentia exeat per retortam. Hanc edulcora, & est quinta Saturni essentia, quam & saccharum vocant." (FIFTH ESSENCE OF LEAD. For two days I roast [i.e., reflux], in embers, lead steeped in distilled vinegar, and the substance [i.e., lead] is dissolved in the dilute vinegar; pour off [the solution] and return [to the reflux flask] the other [i.e., anything that hasn't dissolved] and so proceed until every bit [of lead] has been extracted. Concentrate the collected vinegar into a salt. To this, pour in spirit of wine [i.e., ethanol] [that has been] refluxed or treated with alkali. Reflux for a few days; distill by retort so that the essence [i.e., volatile liquid] is gone. Neutralize this, and [this] is the fifth essence of lead and what they call sugar [of lead].)
- "Aceton - Chemgapedia".
- Dumas, J. (1832) "Sur l'esprit pyro-acétique" (On pyro-acetic spirit), Annales de Chimie et de Physique, 2nd series, 49 : 208–210.
- Liebig, Justus (1832) "Sur les combinaisons produites par l'action du gas oléfiant et l'esprit acétique" (On compounds produced by the action of ethylene and acetic spirit), Annales de Chimie et de Physique, 2nd series, 49 : 146–204 (especially 193–204).
- Bussy, Antoine (1833) "De quelques Produits nouveaux obtenus par l’action des Alcalis sur les Corps gras à une haute température" (On some new products obtained by the action of alkalies on fatty substances at a high temperature), Annales de Chimie et de Physique, 2nd series, 53 : 398–412; see footnote on pp. 408–409.
- Williamson, A. W. (1852) "On Etherification," Journal of the Chemical Society, 4 : 229–239; (especially pp. 237–239).
- Gerhardt, Charles (1853) "Researches sur les acids organiques anhydres" (Research on anhydrous organic acids), Annales de Chimie et de Physique, 3rd series, 37 : 285–342; see p. 339.
- Kekulé, Auguste (1865) "Sur la constitution des substances aromatiques," Bulletin de la Société chimique de Paris, 1 : 98–110; (especially p. 110).
- Kekulé, Auguste (1866) "Untersuchungen über aromatischen Verbindungen" (Investigations into aromatic compounds), Annalen der Chemie und Pharmacie, 137 : 129–196; (especially pp. 143–144).
- Loschmidt, J. (1861) Chemische Studien Vienna, Austria-Hungary: Carl Gerold's Sohn.
- Chaim Weizmann. chemistryexplained.com
- Greiner, Camara; Funada, C (June 2010). "CEH Marketing Research Report: ACETONE". Chemical Economics Handbook. SRI consulting. Retrieved 2 September 2016.(subscription required)
- "Acetone Uses and Market Data". ICIS.com. October 2010. Archived from the original on 2009-05-15. Retrieved 2011-03-21.
- Acetone (US Gulf) Price Report – Chemical pricing information Archived 2013-05-16 at the Wayback Machine. ICIS Pricing. Retrieved on 2012-11-26.
- Wittcoff, M.M.; Green, H.A. (2003). Organic chemistry principles and industrial practice (1. ed., 1. reprint. ed.). Weinheim: Wiley-VCH. p. 4. ISBN 3-527-30289-1.
- Hine, Jack; Arata, Kazushi (1976). "Keto-Enol Tautomerism. II. The Calorimetrical Determination of the Equilibrium Constants for Keto-Enol Tautomerism for Cyclohexanone and Acetone". Bulletin of the Chemical Society of Japan. 49 (11): 3089–3092. doi:10.1246/bcsj.49.3089.
- Cataldo, Franco (1996). "Synthesis of ketonic resins from self-polymerization of acetone, 1 Action of protic and Lewis acids on acetone". Die Angewandte Makromolekulare Chemie. 236 (1): 1–19. doi:10.1002/apmc.1996.052360101.
- V. A. Kargin, V. A. Kabanov, V. P. Zubov, I. M. Papisov (1960): "Polymerisation of acetone". Doklady Akademii Nauk SSSR, volume 134, issue 5, pages 1098–1099. Mi dan24153
- Kawai, Wasaburo (1962). "Polymerization of Acetone". Bulletin of the Chemical Society of Japan. 35 (3): 516A. doi:10.1246/bcsj.35.516a.
- Vujasinovic, M; Kocar, M; Kramer, K; Bunc, M; Brvar, M (2007). "Poisoning with 1-propanol and 2-propanol". Human & Experimental Toxicology. 26 (12): 975–8. doi:10.1177/0960327107087794. PMID 18375643. S2CID 11723110.
- Glew, Robert H (2010). "You Can Get There From Here: Acetone, Anionic Ketones and Even-Carbon Fatty Acids can Provide Substrates for Gluconeogenesis". Nig. J. Physiol. Sci. 25: 2–4. Archived from the original on 2013-09-26. Retrieved 2013-09-01.
- Miller, DN; Bazzano, G (1965). "Propanediol metabolism and its relation to lactic acid metabolism". Ann NY Acad Sci. 119 (3): 957–973. Bibcode:1965NYASA.119..957M. doi:10.1111/j.1749-6632.1965.tb47455.x. PMID 4285478. S2CID 37769342.
- Ruddick, JA (1972). "Toxicology, metabolism, and biochemistry of 1,2-propanediol". Toxicol Appl Pharmacol. 21 (1): 102–111. doi:10.1016/0041-008X(72)90032-4. PMID 4553872.
- Mine Safety and Health Administration (MSHA) – Safety Hazard Information – Special Hazards of Acetylene Archived 2016-01-22 at the Wayback Machine. Msha.gov. Retrieved on 2012-11-26.
- History – Acetylene dissolved in acetone Archived 2015-09-15 at the Wayback Machine. Aga.com. Retrieved on 2012-11-26.
- Carlysle, Felicity (2011-07-26). "TLC the Forensic Way". theGIST. Glasgow Insight Into Science & Technology. Archived from the original on July 30, 2016. Retrieved October 10, 2015.
- Picó, Yolanda, ed. (2012). Chemical Analysis of Food: Techniques and Applications. Elsevier. p. 501. ISBN 9780123848628 – via Google Books.
- Nikolin, B; Imamović, B; Medanhodzić-Vuk, S; Sober, M (May 2004). "High Performance Liquid Chromatography in Pharmaceutical Analyses". Bosnian Journal of Basic Medical Sciences. 4 (2): 5–9. doi:10.17305/bjbms.2004.3405. PMC 7250120. PMID 15629016.
- Dong, Michael W. (2016). Modern HPLC for Practicing Scientists. John Wiley & Sons. ISBN 9780471727897 – via Google Books.
- "A Simplified Guide to Forensic Drug Chemistry" (PDF). National Forensic Science Technology Center. Archived from the original (PDF) on March 21, 2016. Retrieved September 24, 2015.
- Fialkov, Alexander; Steiner, Urs; Lehotay, Steven; Amirav, Aviv (January 15, 2007). "Sensitivity and Noise in GC-MS: Achieving Low Limits of Detection for Difficult Analytes". International Journal of Mass Spectrometry. 260 (1): 31–48. Bibcode:2007IJMSp.260...31F. doi:10.1016/j.ijms.2006.07.002.
- Smith, Michael L.; Vorce, Shawn P.; Holler, Justin M.; Shimomura, Eric; Magluilo, Joe; Jacobs, Aaron J.; Huestis, Marilyn A. (June 2007). "Modern Instrumental Methods in Forensic Toxicology". Journal of Analytical Toxicology. 31 (5): 237–253. doi:10.1093/jat/31.5.237. PMC 2745311. PMID 17579968.
- What is an Azeotrope?. Solvent—recycling.com. Retrieved on 2012-11-26.
- Lozano, A.; Yip, B.; Hanson, R.K. (1992). "Acetone: a tracer for concentration measurements in gaseous flows by planar laser-induced fluorescence". Exp. Fluids. 13 (6): 369–376. Bibcode:1992ExFl...13..369L. doi:10.1007/BF00223244. S2CID 121060565.
- Simpson, Deborah M.; Beynon, Robert J. (2009). "Acetone precipitation of proteins and the modification of peptides". Journal of Proteome Research. 9 (1): 444–450. doi:10.1021/pr900806x. ISSN 1535-3907. PMID 20000691.
- "Cleaning Glassware" (PDF). Wesleyan University. September 2009. Retrieved July 7, 2016.
- Addison, Ault (1998). Studyguide for Techniques and Experiments for Organic Chemistry. Sausalito, CA. p. 310. ISBN 9780935702767.
- Basten, O.; Bandorski, D.; Bismarck, C.; Neumann, K.; Fisseler-Eckhoff, A. (13 December 2009). "Acetonkompression". Der Pathologe (in German). 31 (3): 218–224. doi:10.1007/s00292-009-1256-7. PMID 20012620.
- Leung, C. A. W.; Fazzi, G. E.; Melenhorst, J.; Rennspiess, D.; Grabsch, H. I. (November 2018). "Acetone clearance of mesocolic or mesorectal fat increases lymph node yield and may improve detection of high-risk Stage II colorectal cancer patients" (PDF). Colorectal Disease. 20 (11): 1014–1019. doi:10.1111/codi.14335. PMID 29989291. S2CID 205030844.
- Engbaek, K; Johansen, KS; Jensen, ME (February 1979). "A new technique for Gram staining paraffin-embedded tissue". Journal of Clinical Pathology. 32 (2): 187–90. doi:10.1136/jcp.32.2.187. PMC 1145607. PMID 86548.
- Driessen, W.L.; Groeneveld, W.L. (1969). "Complexes with ligands containing the carbonyl group. Part I: Complexes with acetone of some divalent metals containing tetrachloro‐ferrate(III) and ‐indate(III) anions". Recueil des Travaux Chimiques des Pays-Bas. 88: 77977–988.
- Kilner, C. A.; Halcrow, M. A. (2006). "An unusual example of a linearly coordinated acetone ligand in a six-coordinate iron(II) complex". Acta Crystallographica C. 62 (9): 1107–1109. doi:10.1107/S0108270106028903. PMID 16954630.
- Weiner, Myra L.; Lois A. Kotkoskie (1999). Excipient Toxicity and Safety. p. 32. ISBN 978-0-8247-8210-8.
- Inactive Ingredient Search for Approved Drug Products, FDA/Center for Drug Evaluation and Research
- Likhodii SS; Serbanescu I; Cortez MA; Murphy P; Snead OC; Burnham WM (2003). "Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet". Ann Neurol. 54 (2): 219–226. doi:10.1002/ana.10634. PMID 12891674. S2CID 3213318.
- American Chemistry Council Acetone Panel (September 10, 2003). "Acetone (CAS No. 67-64-1) VCCEP Submission" (PDF). pp. 6, 9. Retrieved 2018-04-14.
- "Quality Finish 3D Prints with Acetone". instructables.com
- Acetone MSDS. Hazard.com (1998-04-21). Retrieved on 2012-11-26.
- Basic Information on Acetone. Ccohs.ca (1999-02-19). Retrieved on 2012-11-26.
- "SIDS Initial Assessment Report: Acetone" (PDF). Environmental Protection Agency. Archived from the original (PDF) on 2014-03-09. Retrieved 2014-09-11.
{{cite journal}}: Cite journal requires|journal=(help) - "What are the potential health effects of acetone?". Canadian Centre for Occupational Health and Safety. Archived from the original on 2008-10-17. Retrieved 2008-10-21.
- Safety (MSDS) data for propanone Archived 2018-03-16 at the Wayback Machine. sciencelab.com/msds. Retrieved on 2018-03-19
- Acetone, Agency for Toxic Substances and Disease Registry ToxFAQs, 1995
- Darwent, B. deB.; Allard, M. J.; Hartman, M. F.; Lange, L. J. (1960). "The Photolysis of Acetone". Journal of Physical Chemistry. 64 (12): 1847–1850. doi:10.1021/j100841a010.
- "Safety Data Sheet Acetone" (PDF). J.M. Loveridge. Archived from the original (PDF) on 2009-03-20. Retrieved 2012-11-26.
- Jordans, Frank (30 July 2015). "Philae probe finds evidence that comets can be cosmic labs". The Washington Post. Associated Press. Archived from the original on 23 December 2018. Retrieved 30 July 2015.
- "Science on the Surface of a Comet". European Space Agency. 30 July 2015. Retrieved 30 July 2015.
- Bibring, J.-P.; Taylor, M.G.G.T.; Alexander, C.; Auster, U.; Biele, J.; Finzi, A. Ercoli; Goesmann, F.; Klingehoefer, G.; Kofman, W.; Mottola, S.; Seidenstiker, K.J.; Spohn, T.; Wright, I. (31 July 2015). "Philae's First Days on the Comet – Introduction to Special Issue". Science. 349 (6247): 493. Bibcode:2015Sci...349..493B. doi:10.1126/science.aac5116. PMID 26228139.
External links
- International Chemical Safety Card 0087
- NIOSH Pocket Guide to Chemical Hazards
- Acetone Safety Data Sheet (SDS)
- Hazardous substances databank entry at the national library of medicine Archived 2018-12-04 at the Wayback Machine
- SIDS Initial Assessment Report for Acetone from the Organisation for Economic Co-operation and Development (OECD)
- Calculation of vapor pressure, liquid density, dynamic liquid viscosity, surface tension of acetone